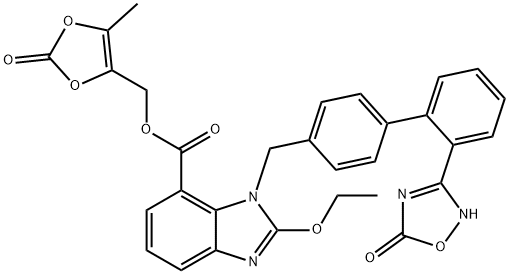
Azilsartan medoxomil (Edarbi), an angiotensin II receptor antagonist,
was approved by the U.S. FDA in February 2011 for the treatment of
hypertension in adults. The discovery of azilsartan was the result
of a medicinal chemistry effort to identify an ARB with a different carboxylic
acid isostere than the ones found in the marketed ARBs. Several of the
marketed ARBs use a tetrazole group as a carboxylic acid isostere. The medicinal
chemistry approach that led to azilsartan involved the replacement of
this commonly used tetrazole with a 5-oxo-1,2,4-oxadiazole group.
Azilsartan can be synthesized by Suzuki coupling of p-tolyl boronic acid
to 2-bromobenzonitrile, followed by bromination of the methyl group.
The bromide is displaced to introduce a protected 2-ethoxy-1H-benzo[d]
imidazole-7-carboxylate. The cyano group is converted to a hydroxylamidine,
followed by reaction with an alkyl-chloroformate and intramolecular
cyclization to form the 5-oxo-1,2,4-oxadiazole ring. The acid is
then deprotected and converted to a prodrug. The parent, azilsartan has been
extensively characterized in vitro and compared with other marketed AT1
antagonists olmesartan, valsartan, telmisartan, irbesartan, and candesartan.
Azilsartan was found to be a potent (IC50=2.6 nM), selective, inverse agonist
of the AT1 receptor. From washout experiments, azilsartan was found
have slow dissociation from the receptor and thus is characterized as an insurmountable
antagonist.