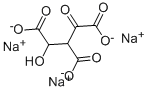
Aconitase catalyzes the stereospecific isomerization of citrate to isocitrate in the first step of the citric acid cycle. It acts as an iron regulatory protein (IRP), controlling the translation of proteins involved in iron metabolism when iron is scarce and resumes aconitase activity when iron is abundant. Aconitase is now recognized as a regulator of iron-induced glutamate production. Oxalomalic acid, formed by the condensation of oxaloacetate with glyoxylate in vivo, inhibits both aconitase and NADP-dependent isocitrate dehydrogenase in the conversion of citrate to isocitrate at concentrations as low as 1 mM. At 5 mM, oxalomalic acid inhibition of aconitase leads to a decrease in the binding activity of IRP1 and a decrease in glutamate secretion in cultured lens epithelial cells, retinal pigment epithelial cells, and neurons.