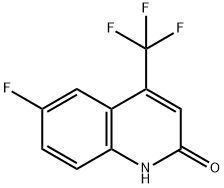
As a quinoline derivative, 6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline is substituted with trifluoromethyl, hydroxyl, and fluoride groups. In particular, 6-Fluoro-4-hydroxy-2-(trifluoromethyl)quinoline can exist in a tautomeric form, known as quinolone, where the hydroxy group becomes a ketone and the imine changes to an amine. This compound is a precursor for synthesizing thioquinolines, which are used to develop non-cytotoxic, potent and selective antitubercular agents. The thiolation reaction is carried out using phosphorus pentasulfide in pyridine.