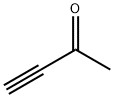
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (+/-)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones. 3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines2. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives. 3-Butyn-2-one, is used as a reactant with Dimethyl- acetone-1,3-dicarboxyl-ate, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process.