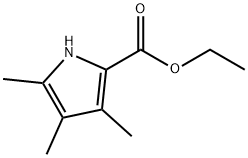
ETHYL 3,4,5-TRIMETHYLPYRROLE-2-CARBOXYLATE
$29.8 - $114.65
- Product nameETHYL 3,4,5-TRIMETHYLPYRROLE-2-CARBOXYLATE
- Cas No2199-46-4
- MFC10H15NO2
- MW181.23
- MDL NumberMFCD00051948
| Brand | Packaging | Price | Product name | |
|---|---|---|---|---|
|
Sigma-Aldrich
S762768
|
250MG | $29.8 | ETHYL 3,4,5-TRIMETHYL-2-PYRROLECARBOXYLATE |
|
|
Sigma-Aldrich
S762768
|
1 unit | $30.7 | ETHYL 3,4,5-TRIMETHYL-2-PYRROLECARBOXYLATE |