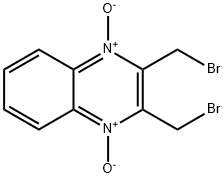
Peroxiredoxins are a widely conserved family of enzymes that function in antioxidant defense and act in redox signaling pathways. Increased expression of human peroxiredoxin is associated with cancer, cardiovascular dysfunction, and neurodegeneration. Conoidin A inactivates peroxiredoxins by covalently binding to the catalytic cysteine on the enzyme. It has been shown to inhibit peroxiredoxin II (IC50 = 23 μM) in the parasite T. gondii and peroxiredoxin I in the hookworm A. ceylanicum. At 5 μM, conoidin A can also inhibit the glucose oxidase-mediated hyperoxidation of mammalian peroxiredoxin I and II, but not peroxiredoxin III.