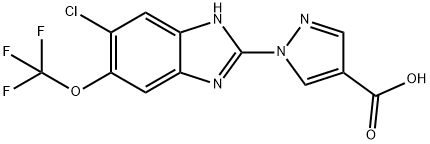
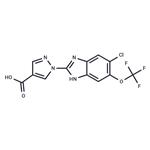
Hypoxia-inducible factor (HIF) prolyl hydroxylase (PHD) enzymes act as central gatekeepers of posttranscriptional and transcriptional adaptation to hypoxia, oxidative stress, and excitotoxicity. Their catalytic activity is dependent on iron, 2-oxoglutarate (2-OG), and oxygen, and leads to the stabilization of a host of proteins, including the hypoxia-inducible transcription factors in response to oxygen availability. Inhibitors of PHD have been used to mimic a hypoxic response in order to study a range of oxygen-deprivation-related disorders, including anemia, ulcerative colitis, myocardial ischemia, stroke, and metabolic disorders. JNJ-42041935 is a selective, 2-OG competitive, and reversible inhibitor of PHD enzymes (pKis = 7.91, 7.29, and 7.65 for PHD1, 2, and 3, respectively). It is >100-fold selective for PHD compared to the related FIH (factor-inhibiting HIF) and a panel of various other enzymes. In an inflammation-induced anemia model in rats, 100 μM/kg/day JNJ-42041935 significantly increased the number of circulating reticulocytes and red blood cells, increased blood hemoglobin and hematocrit, and restored mean corpuscular volume and mean cell hemoglobin of red bloods cells.