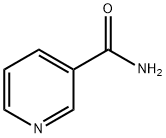
-
물성
함량이품목을건조물로환산한것은니코틴산아미드(C6H6N2O) 98.5~101.0%를함유한다.
성상이품목은백색의결정성분말로서냄새가없고 쓴맛을가지고있다.
-
제품 소개
(1) 이 품목의 수용액(1→10)은 중성이다.
(2) 이 품목 10mg을 백금판상에서 태우면 피리딘의 냄새가 난다.
(3) 이 품목 20mg에 수산화나트륨시액 5mL를 가하여 조용히 끓이면 암모니아 냄새가 난다.
-
제품 소개
(1) 융점 : 이 품목의 융점은 128~131℃이어야 한다.
(2) 액성 : 이 품목 1g을 취해 물을 가해 20mL로 한 액의 pH는 6.0~7.5이다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(4) 황산정색물 : 이 품목 0.2g을 취하여 황산정색물시험법에 따라 시험할 때, 그 액은 비색표준용액 A보다 진하여서는 아니 된다.
건조감량 이 품목을 감압데시케이타(실리카겔)에서 4시간 건조할 때, 그 감량은 0.5% 이하이어야 한다.
강열잔류물 이 품목의 강열잔류물은 0.1% 이하이어야 한다.
정 량 법 이 품목 약 0.2g을 정밀히 달아 빙초산(비수적정용) 30mL를 가하고 필요하면 가온하여 녹이고 식힌 후 톨루엔 100mL를 가한 다음 0.1N 과염소산용액으로 적정한다(지시약 : 크리스탈바이올렛․빙초산시액 2방울). 종말점은 액의 자색이 청색을 지나 녹색으로 변하는 점이다. 같은 방법으로 공시험을 한다.
-
순도시험
순도시험 (1) 융점 : 이 품목의 융점은 128~131℃이어야 한다.
(2) 액성 : 이 품목 1g을 취해 물을 가해 20mL로 한 액의 pH는 6.0~7.5이다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(4) 황산정색물 : 이 품목 0.2g을 취하여 황산정색물시험법에 따라 시험할 때, 그 액은 비색표준용액 A보다 진하여서는 아니 된다.
-
확인시험
(1) 이 품목의 수용액(1→10)은 중성이다.
(2) 이 품목 10mg을 백금판상에서 태우면 피리딘의 냄새가 난다.
(3) 이 품목 20mg에 수산화나트륨시액 5mL를 가하여 조용히 끓이면 암모니아 냄새가 난다.
-
정량법
이 품목 약 0.2g을 정밀히 달아 빙초산(비수적정용) 30mL를 가하고 필요하면 가온하여 녹이고 식힌 후 톨루엔 100mL를 가한 다음 0.1N 과염소산용액으로 적정한다(지시약 : 크리스탈바이올렛․빙초산시액 2방울). 종말점은 액의 자색이 청색을 지나 녹색으로 변하는 점이다. 같은 방법으로 공시험을 한다.
0.1N 과염소산용액 1mL = 12.21mg C6H6N2O
-
강열잔류물
이 품목의 강열잔류물은 0.1% 이하이어야 한다.
-
개요
A white, crystalline powder. It is odorless or nearly so, and has a bitter taste. Its solutions are neutral to litmus. One g dissolves in about 1 mL of water, in about 1.5 mL of alcohol, and in about 10 mL of glycerin.
-
화학적 성질
Niacinamide (Nicotinamide) is a white crystalline powder
or forms colorless needle-like crystals.
-
용도
Niacinamide is a nutrient and dietary supplement that is an available form of niacin. Nicotinic acid is pyridine beta-carboxylic acid and nicotinamide, which is another term for niacinamide, is the corresponding amide. It is a powder of good water solubility, having a solubility of 1 g in 1 ml of water. Unlike niacin, it has a bitter taste; the taste is masked in the encapsulated form. Used in fortification of cereals, snack foods, and powdered beverages. niacinamide is used as a skin stimulant and skin smoother. Niacinamide USP is used as food additive, for multivitamin preparations and as intermediate for pharmaceuticals and cosmetics.
-
정의
ChEBI: A pyridinecarboxamide that is pyridine in which the hydrogen at position 3 is replaced by a carboxamide group.
-
일반 설명
Vitamin B3 was formerly called nicotinic acid; however, the term niacin is now preferred to avoid any confusion with the alkaloid, nicotine. Niacinamide, also known as nicotinamide, refers to the amide derivative of niacin that is equivalent in vitamin activity. Some texts use niacin to refer to nicotinic acid, niacinamide, and any derivatives with vitamin activity comparable to niacin. Furthermore, research and chemistry-based resources use the terms nicotinic acid and nicotinamide; whereas pharmacy resources use niacin and niacinamide.
-
공기와 물의 반응
Water soluble.
-
반응 프로필
An amine and amide. Acts as a weak base in solution. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
-
Clinical Use
Niacin is used in the treatment of niacin deficiency, which is referred to as pellagra (from the Italian, pelle for “skin” and agra for “dry”). The major systems affected are the gastrointestinal tract (diarrhea, enteritis and stomatitis), the skin (dermatitis), and the CNS (generalized neurological deficits including dementia). Pellagra has become a rare condition in the United States and other countries that require or encourage enrichment of wheat flour or fortification of cereals with niacin. Because the nucleotide form can be synthesized in vivo from tryptophan, pellagra is most often seen in areas where the diet is deficient in both niacin and tryptophan. Typically, maize (corn)-based diets meet this criteria. Niacin deficiency can also result from diarrhea, cirrhosis, alcoholism, or Hartnup disease. It is interesting that niacin deficiency can also, rarely, result from vitamin B6 deficiency (see Vitamin B6 section). Niacin, but not niacinamide, is also one of the few vitamins that are useful in the treatment of diseases unrelated to deficiencies.
-
Safety Profile
Nicotinamide is a safe and inexpensive compound with negligible side effects. It is well tolerated even in doses of 1g/day to 3g/day.There are no reports of teratogenicity with nicotinamide. Minor side effects include nausea, vomiting, headache, fatigue. It does not cause vasodilatory side effects like flushing, alteration in blood pressure, body temperature or pulse as seen with niacin.In topical formulation, it does not cause skin irritation, photosensitization in concentrations of 0.0001% to 4%.
-
잠재적 노출
Used as a dietary supplement and
food additive.
-
신진 대사
Nicotinamide is ingested in food as part of pyridine nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) in plant and animal tissues. After the co?enzymes have separated, nicotinamide is absorbed almost completely in the small intestine. After absorption, nicotinamide is stored as NAD in the liver and excretion occurs via kidneys. Tryptophan is converted to nicotinamide through kynurenine?anthranilate pathway in the liver. Tryptophan can thus satisfy the requirement for dietary nicotinic acid.
-
Purification Methods
Crystallise niacin from *benzene. It has solubility in g/ml: H2O (1), EtOH (0.7) and glycerol (0.1). [Methods in Enzymology 66 23 1980, UV: Armarego Physical Methods in Heterocyclic Chemistry (Ed Katritzky, Academic Press) Vol III 83 1971, Beilstein 22 III/IV 389, 22/2 V 80.]
-
비 호환성
Combustible solid; dust may form explosive
mixture with air. Amides are incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides.