-
外観
無色〜暗赤褐色, 澄明の液体
-
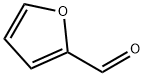
定義
本品は、次の化学式で表される環状アルデヒドである。
-
性質
フルフラールの融点は-36.5°C、沸点は161.7°Cであり、引火点は62°Cです。無色油状の液体ですが、空気に触れるとすぐ黄色く変色します。
フルフラールはアーモンドに似た香気を有します。エーテルやアルコールなどの、多くの有機溶剤に溶けますが、アルカン類や水には微溶です。
一般的な芳香族化合物やアルデヒド類と同様の化学反応を起こします。ただし、ベンゼンほど安定しておらず、ほかの芳香族化合物と比べて化学反応を起こしやすいです。
例えば、フルフラールを250°C以上に熱すると、一酸化炭素とフランに分解します。酸とともに加熱すると、熱硬化性樹脂になり固まります。強い酸や塩基との反応によって、火災や爆発の危険性もあるため、取り扱いには注意が必要です。
-
溶解性
エタノール及びアセトンに極めて溶けやすく、水にやや溶けやすい。
-
解説
2-フルアルデヒド,2-furancarboxaldehyde.C5H4O2(96.09).フルフラールともいう.ペントサンを含む植物体(トウモロコシの穂軸,麬(ふすま)など)を希硫酸と加熱しながら蒸留すると得られる.融点-38.7 ℃,沸点161.7 ℃.d2041.1594.n20D1.5261.水に可溶,エタノール,エーテルに易溶.ベンズアルデヒドに似た性質をもつ.空気中,光にさらすと黄~茶色となり,樹脂化する.用途はフェノール-フラン樹脂,ナイロン66の原料,溶剤,浮遊選鉱剤,除草剤,殺菌剤など.催涙性があり,粘膜を刺激する.LD50 50~100 mg/kg(ラット,経口).
-
用途
溶剤、フラン樹脂原料、潤滑油精製、医薬品原料
-
用途
ナイロン原料、潤滑油
-
構造
フルフラールは、フランの2位がホルミル基で置換された構造を有します。示性式は(C4H3O)CHOで表され、モル質量は96.09g/mol、密度は1.16g/mLです。
-
化粧品の成分用途
溶剤、香料
-
合成
多くの植物には、多糖類やヘミセルロースが含まれています。そのため、希硫酸とともに熱すると、ヘミセルロースの加水分解によって、キシロースのような糖類に変化します。同条件下でキシロースなどのC5糖類の脱水によって、3個の水分子を放出し、フルフラールを生成可能です。希硫酸の代わりに希塩酸を使用する方法もあります。
トウモロコシや燕麦殻の場合には、収率は比較的高く、20%ほどです。穀物の籾殻を用いると、原料のおよそ10%のフルフラールが得られます。水とともにフルフラールが蒸発するため、分離回収して濃縮できます。
-
危険性
フルフラールに触れた際には、気道や皮膚が刺激を受けて、水が肺にたまる場合もあります。フルフラールを吸い込んだり、呑み込んだりした場合には、頭痛、酔い、吐き気、めまい、涙目などの中毒症状を起こす可能性があります。意識不明や死に至るケースもあるため、注意が必要です。
-
使用上の注意
不活性ガス封入
-
説明
Furfural is a colourless to amber-like oily liquid with an almond-like odour. On exposure
to light and air, it turns reddish brown. Furfural is used in making chemicals,
as a solvent in petroleum refining, a fungicide, and a weed killer. It is incompatible with strong acids, oxidisers, and strong alkalis. It undergoes polymerisation on contact
with strong acids or strong alkalis. Furfural is produced commercially by the acid
hydrolysis of pentosan polysaccharides from non-food residues of food crops and wood
wastes. It is used widely as a solvent in petroleum refining, in the production of phenolic
resins, and in a variety of other applications. Human exposure to furfural occurs during
its production and use, as a result of its natural occurrence in many foods and from the
combustion of coal and wood.
-
化学的特性
Furfural is a colorless to yellow aromatic het erocyclic aldehyde with an almond-like odor. Turns amber
on exposure to light and air.
-
物理的性質
Colorless to yellow liquid with an almond-like odor. Turns reddish brown on exposure to light and
air. Odor and taste thresholds are 0.4 and 4 ppm, respectively (quoted, Keith and Walters, 1992).
Shaw et al. (1970) reported a taste threshold in water of 80 ppm.
-
天然物の起源
Reported found in several essential oils from plants of the Pinaceae family, in the essential oil from Cajenne linaloe, in the oil from leaves of Trifolium pratense and Trifolium incarnatum, in the distillation waters of several essential oils, such as ambrettee and angelica seeds, in Ceylon cinnamon essential oil, in petitgrain oil, ylang-ylang, lavender, lemongrass, calamus, eucalyptus, neroli, sandalwood, tobacco leaves and others Also reported found in many foods including apple, apricot, citrus peel oils and juices, berries, guava, grapes, pineapple, asparagus, kohlrabi, celery, onion, leek, potato, tomato, cinnamon, mustard, bread, cheeses, meats, fsh, cognac, rum, whiskies, cider, grape wine, cocoa, coffee, tea, barley, peanuts, popcorn, pecans, oats, honey, soybeans, passion fruit, plums, mushroom, mango, tamarind, fruit brandies, whiskey malt, white bread, rum, bourbon, cardamom, coriander seed, calamus, corn oil, malt, wort and other sources
-
使用
In the manufacture of furfural-phenol plastics such as Durite; in solvent refining of petroleum oils; in the preparation of pyromucic acid. As a solvent for nitrated cotton, cellulose acetate, and gums; in the manufacture of varnishes; for accelerating vulcanization; as insecticide, fungicide, germicide; as reagent in analytical chemistry. In the synthesis of furan derivatives.
-
定義
furfural: A colourless liquid,C5H4O2, b.p. 162°C, which darkenson standing in air. It is the aldehydederivative of furan and occurs invarious essential oils and in fuseloil. It is used as a solvent for extractingmineral oils and natural resinsand itself forms resins with somearomatic compounds.
-
製造方法
Industrially prepared from pentosans that are contained in cereal straws and brans; these materials are previously digested with diluted H2SO4, and the formed furfural steam is distilled.
-
調製方法
Furfural is obtained commercially by treating pentosan-rich agricultural residues (corncobs, oat hulls, cottonseed hulls, bagasse, rice hulls) with a dilute acid and removing the furfural by steam distillation. Major industrial uses of furfuraldehyde include: (1) the production of furans and tetrahydrofurans where the compound is an intermediate; (2) the solvent refining of petroleum and rosin products; (3) the solvent binding of bonded phenolic products; and (4) the extractive distillation of butadiene from other C4 hydrocarbons.
When pentoses, e.g., arabinose, xylose, are heated with dilute HCl, furfuraldehyde is formed, recognizable by deep red coloration with phloroglucinol, or by the formation, with phenylhydrazine, of furfuraldehyde phenylhydrazone C4H3O·CH : NNHC6H5, solid, mp 97 °C.
-
反応性
Aside from a darkening in color, furfural is relatively stable thermally and does not exhibit changes in physical properties after prolonged heating up to 230°C. The reactions of furfural are typical of those of the aromatic aldehydes, although some complex side reactions occur because of the reactive ring. Furfural yields acetals, condenses with active methylene compounds, reacts with Grignard reagents, and provides a bisulfite complex. Upon reduction, furfural yields furfural alcohol; upon oxidation, it yields furoic acid. It can be decarbonylated to furan.
-
一般的な説明
Colorless or reddish-brown mobile liquids with a penetrating odor. Flash points 140°F. Denser than water and soluble in water. Vapors heavier than air. May be toxic by ingestion, skin absorption or inhalation.
-
空気と水の反応
Flammable. Furfural is sensitive to light and air. Soluble in water, with mixing.
-
反応プロフィール
Furfural reacts with sodium hydrogen carbonate. Furfural also can react with strong oxidizers. An exothermic resinification of almost explosive violence can occur upon contact with strong mineral acids or alkalis. Furfural forms condensation products with many types of compounds, including phenol, amines and urea. .
-
危険性
Absorbed by skin; irritant to eyes, skin,
and mucous membranes. Toxic by skin absorption;
questionable carcinogen.
-
健康ハザード
Vapor may irritate eyes and respiratory system. Liquid irritates skin and may cause dermatitis.
-
火災危険
Special Hazards of Combustion Products: Irritating vapors are generated when heated
-
使用用途
フルフラールは、熱硬化性、耐食性、物理的耐性などの優れた特性を備えており、樹脂の原料として利用されます。例えば、耐薬・耐熱性表面処理剤として用いられているフラン樹脂が挙げられます。
また、フルフラールは、アルコール、エステル、などの有機溶剤に混ざるため、溶剤として広く利用されています。主に潤滑油や脱色剤の精製に使用可能です。その他、除草剤や殺虫剤としても利用されます。
-
工業用途
Also known as furfuraldehyde, furol, and pyromuclealdehyde,furfural is a yellowish liquidwith an aromatic odor, soluble in water and inalcohol, but not in petroleum hydrocarbons. Onexposure, it darkens and gradually decomposes.Furfural occurs in different forms in variousplant life and is obtained from complex carbohydratesknown as pentosans, which occur insuch agricultural wastes as cornstalks, corncobs,straw, oat husks, peanut shells, bagasse,and rice. Furfural is used for making syntheticplastics, as a plasticizer in other synthetic resins,as a preservative in weed killers, and as aselective solvent especially for removing aromaticand sulfur compounds from lubricatingoils. It is also used for the making of butadiene,adiponitrile, and other chemicals.
Various derivatives of furfural are not used,and these, known collectively as furans, are nowmade synthetically from formaldehyde andacetylene, which react to form butyl nedole.
-
安全性プロファイル
Confirmed carcinogen.
Poison by ingestion, intraperitoneal,
subcutaneous, intravenous, and
intramuscular routes. Moderately toxic by
inhalation and sktn contact. Human
mutation data reported. A skin and eye
irritant. Mutation data reported. The liquid is
dangerous to the eyes. The vapor is irritating
to mucous membranes and is a central
nervous system poison. However, its low
volatility reduces its toxicity effect. Ingestion
of furfural has produced cirrhosis of the
liver in rats. In industry there is a tendency
to minimize the danger of acute effects
resulting from exposure to it. This is
particularly true because of its low volathty. Flammable liquid when exposed to heat or
flame; can react with oxidizing materials.
Moderate explosion hazard when exposed
to heat or flame or by chemical reaction. An
exothermic polymerization of almost
explosive violence can occur upon contact
with strong mineral acids or alkalies. Keep
away from heat and open flames. Mixture
with sodium hydrogen carbonate ignites
spontaneously. To fight fire, use alcohol
foam, CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes.
-
職業ばく露
Furfural is used for lube oil refining
and butadiene extraction; as a solvent for wood resin,
nitrated cotton, cellulose acetate, and gums; in the produc tion of phenolic plastics, thermosetting resins, refined
petroleum oils, dyes, and varnishes; in the manufacture of
pyromucic acid, vulcanized rubber, insecticides, fungicides,
herbicides, germicides, furan derivatives, polymers, and
other organic chemicals.
-
概要
フルフラールとは、化学式がC5H4O2で表される、芳香族アルデヒドの1種です。
2-フランカルボキシアルデヒドとも呼ばれます。1832年にヨハン・オルフガング・デーベライナー (英: Johann Döbereiner) によって、初めてギ酸の副産物として分離されました。
工業的には、トウモロコシの芯やサトウキビのバガスなどの農業副産物から、世界中で数十万トンが生産されています。原料を高圧蒸気下で処理した後、蒸留によって回収し、水とフルフラールを分離可能です。バイオマス由来の安価で再生可能な非石油ベースの化学原料として注目されています。
-
発がん性
The IARC evaluated furfural
and determined that there was inadequate evidence in
humans for the carcinogenicity of furfural. There is limited
evidence in experimental animals for the carcinogenicity of
furfural.
-
環境運命予測
Biological. Under nitrate-reducing and methanogenic conditions, furfural biodegraded to
methane and carbon dioxide (Knight et al., 1990). In activated sludge inoculum, following a 20-d
adaptation period, 96.3% COD removal was achieved. The average rate of biodegradation was
37.0 mg COD/g?h (Pitter, 1976).
Photolytic. Atkinson (1985) reported an estimated photooxidation half-life of 10.5 h for the
reaction of furfural with OH radicals in the atmosphere.
Chemical/Physical. Slowly resinifies at room temperature (Windholz et al., 1983). May
polymerize on contact with strong acids or strong alkalies (NIOSH, 1997).
-
輸送方法
UN1199 Furaldehyde, Hazard class: 6.1; Labels:
6.1-Poisonous materials, 3-Flammable liquid.
-
不和合性
May form explosive mixture with air.
Acids and bases can cause polymerization, causing fire or explosion hazard. Reacts violently with oxidants.
Incompatible with strong acids; caustics, ammonia, ali phatic amines; alkanolamines, alromatic amines; oxidizers.
Attacks many plastics.
-
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo) must
conform with EPA regulations governing storage, transpor tation, treatment, and waste disposal.