-
性質
水素化リチウムの融点は680°Cとなり、720°Cで分解します。水素化リチウムの特性としてにはわずかに溶けます。
アルカリ金属の水素化物中で、最も安定です。常温の乾燥空気中では分解しませんが、光に当たると分解して黒くなります。水素化リチウムはアルコール類とも反応します。
-
反応
水素化リチウムは水分と激しく反応し、水素と水酸化リチウムが生成します。水やプロトン性試薬に対して、非常に反応性が高いです。水素化リチウムの粉末は低湿度の空気と急速に反応すると、LiOH、Li2O、Li2CO3を形成します。湿った空気中で粉末は自然発火して、窒素化合物を含む生成物の混合物を生じます。
水素化リチウムと二酸化硫黄が反応すると、亜ジチオン酸リチウムを生成可能です。アセチレンとの反応では、炭化リチウムと水素になります。無水有機酸、フェノール、酸無水物ともゆっくり反応し、水素ガスと酸のリチウム塩を生成します。塩化アルミニウムとの反応によって、水素化アルミニウムリチウム (LiAlH4) を合成可能です。
-
解説
LiH(7.95).溶融Liに700~900 ℃ で水素ガスを反応させてつくる.ヒドリドリチウム, 立方晶系結晶で岩塩型構造.Li-H2.04 Å.純粋な白色であるが,貯蔵により黒変するため,市販品は普通灰色.水素化リチウムは,密度0.77 g cm-3.融点680 ℃.一般に溶媒に難溶であるが,ジエチルエーテルに微溶.水とはげしく反応して H2 を発生し,LiOHとなる.アルコール類とも反応する.NH3とは高温で反応してリチウムアミドをつくる.室温,乾燥空気中では安定で,高温では,Cl2,O2,N2 などとも反応する.AlCl3と反応して,有機(薬品)合成工業に重要な還元剤LiAlH4となる.ハロゲン化シランの還元剤として,半導体用のシラン合成に使われる.加水分解で大量の水素が発生するため,野外などで水素発生源(風船・気球用など)に用いられる.[CAS 7580-67-8]
森北出版「化学辞典(第2版)
-
用途
有機合成の還元剤,高純度シリコン製造用 (NITE CHRIP)
-
構造
水素化リチウムの化学式はLiHで表記されます。式量は7.95であり、密度は0.82g/cm3です。
水素雰囲気下で金属リチウムを赤熱すると、無色の立方晶系のイオン結晶が得られます。H-とLi+から構成される塩化ナトリウム型構造を取っており、Li−H間の距離は2.04Åです。
-
合成法
水素化リチウムは溶融状態のリチウムに水素ガスを反応させて製造されます。600°C以上で急速に反応が進みます。0.001~0.003%の炭素の添加や圧力の上昇によって、2時間で収率を最大98%まで増加可能です。ただし収率は水素化リチウムの表面状態に大きく依存します。
水素化アルミニウムリチウム、水素化ホウ素リチウム、n-ブチルリチウム、エチルリチウムなどの熱分解によっても、水素化リチウムは生じます。
-
危険性
水素化リチウムは湿気の多い空気中で爆発しやすいです。乾燥した空気中でも、静電気によって爆発する可能性があります。空気中の濃度が5~55mg/m3の水素化リチウムの粉塵は、粘膜や皮膚を刺激し、アレルギー反応を引き起こす場合があります。
水素化リチウムが反応して生じる一部のリチウム塩は有毒です。通常、特定のプラスチック、セラミック、鋼で作られた容器を使用して油中で輸送され、乾燥したアルゴンやヘリウムの雰囲気で処理されます。
参考文献
-
化学的特性
Lithium hydride (LiH) is a crystalline salt substance(face-centered cubic) that is white in its pure form, As an engineering material, it has properties of interest in many technologies. For example,the high hydrogen content and light weight of LiH make it useful for neutron shields and moderators in nuclear power plants. In addition, the high heat of fusion combined with light weight make LiH appropriate for heat storage media for solar power plants on satellites and may be used as a heat sink for different applications. Typically, processes for production of LiH involve handling of LiH at temperatures above its meltingpoint (688 DC). Type 304L stainless steel is utilized for many process components handling molten LiH.
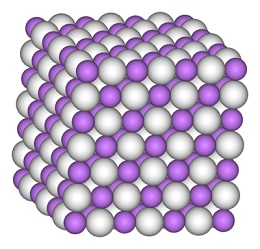
Lithium hydride is a typical ionic hydride with lithium cations and hydride anions. Electrolysis of molten material results in formation of lithium metal at the cathode and hydrogen at the anode. The lithium hydride-water reaction, which results in the release of hydrogen gas, is also indicative of a negatively charged hydrogen.
-
物理的性質
White crystalline solid; cubic crystals; density 0.82 g/cm3; melts at 686.4°C; decomposes in water; soluble in acids.
-
使用
Lithium hydride is used in the manufactureof lithium aluminum hydride and silane, as apowerful reducing agent, as a condensationagent in organic synthesis, as a portablesource of hydrogen, and as a lightweight nuclear shielding material. It is now beingused for storing thermal energy for spacepower systems (Morris et al. 1988).
-
定義
lithium hydride: A white solid,LiH; cubic; r.d. 0.82; m.p. 680°C; decomposesat about 850°C. It is producedby direct combination of theelements at temperatures above500°C. The bonding in lithium hydrideis believed to be largely ionic;i.e. Li+H- as supported by the factthat hydrogen is released from theanode on electrolysis of the moltensalt. The compound reacts violentlyand exothermically with water toyield hydrogen and lithium hydroxide.It is used as a reducing agent toprepare other hydrides and the 2Hisotopic compound, lithiumdeuteride, is particularly valuable fordeuterating a range of organic compounds.Lithium hydride has alsobeen used as a shielding material forthermal neutrons.
-
製造方法
Lithium hydride is prepared by heating lithium metal with hydrogen above 440°C. The reaction is exothermic and can be controlled once it is initiated, without external heating. The heat of formation is greater than that of sodium hydride:
2Li + H2 → 2LiH
-
反応性
Lithium hydride reacts vigorously with water, forming lithium hydroxide with the evolution of hydrogen:
LiH + H2O → LiOH + H2
The hydride also reacts with ammonia forming lithium amide and evolving hydrogen:
LiH + NH3 → LiNH2 + H2
Lithium hydride is a strong reducing agent and would, therefore, react with compounds that contain oxygen. Even many highly stable oxides of metals and nonmetals can be reduced. It reduces metal oxides to metals and carbon dioxide to carbon:
Fe3O4 + 4LiH → 3Fe + 4NaOH
2LiH + CO2 → Li2O + C + H2O
It undergoes violent reactions with oxidizing agents.
Lithium hydride reacts with aluminum hydride forming lithium aluminum hydride, a powerful reducing agent:
LiH + AlH3 → LiAlH4
Lithium hydride consisting of Li+ and H– ions exhibits properties of an ionic salt, both cationic and anionic; such as a strong electrolyte. Thus, when electrolyzed at temperatures slightly below its melting point, it dissociates to Li+ and H¯ ions. Hydrogen gas is liberated at the anode.
The hydride ion, H:¯ being a strong base, would react with alcohols, forming alkoxides and liberating hydrogen:
CH3CH2OH + LiH → CH3CH2OLi + H2
(ethanol) (lithium ethoxide)
(CH3)3COH + LiH → (CH3)3COLi + H2
(tert-butanol) (lithium tert-butoxide)
-
一般的な説明
A white or translucent crystalline mass or powder. The commercial product is light bluish-gray lumps due to the presence of minute amounts of colloidally dispersed lithium.
-
空気と水の反応
Burns readily in air, particularly if powdered. May ignite spontaneously in moist air. Reacts rapidly with water to form caustic lithium hydroxide and hydrogen [Bretherick 1979 p. 107].
-
反応プロフィール
Lithium hydride is a strong reducing agent. May decompose violently in contact with most oxidizing materials. Reacts exothermically with water to form caustic lithium hydroxide and hydrogen gas; the hydrogen may ignite. May ignite spontaneously in moist air. Mixtures with liquid oxygen are explosive. Ignites on contact with dinitrogen oxide [Mellor, 1967, vol. 8, suppl. 2.2, p. 214].
-
健康ハザード
The health hazard due to lithium hydride maybe attributed to the following properties: (1)corrosivity of the hydride, (2) its hydrolysisto strongly basic lithium hydroxide, and (3)toxicity of the lithium metal. However, thelatter property, which may arise becauseof the formation of lithium resulting fromthe decomposition of lithium hydride andthe metabolic role of lithium, is not yetestablished.
This compound is highly corrosive to skin.Contact with eyes can produce severe irritationand possible injury. It can hydrolyzewith body fluid, forming lithium hydroxide,which is also corrosive to the skin andharmful to the eyes. Animal tests indicatedthat exposure to its dust or vapor at a levelexceeding 10 mg/m3 eroded the body fur andskin, caused severe inflammation of the eyes,and led to the destruction of external nasalseptum (ACGIH 1986). No chronic effectswere observed.
-
火災危険
In a fire, irritating alkali fumes may form. Lithium hydride can form airborne dust clouds which may explode on contact with flame, heat, or oxidizing materials. Additionally, spontaneous ignition occurs when nitrous oxide and Lithium hydride are mixed. Lithium hydride also forms explosive mixtures with liquid oxygen. Contact with heat, moisture or acid causes exothermic reaction and evolution of hydrogen as well as lithium hydroxide. Incompatible with air and moisture, nitrous oxide, strong oxidizers, and liquid oxygen. Lithium hydride may ignite spontaneously in air and should be maintained and handled out of contact with air and moisture. Any contact with nitrous oxide; airborne powders may ignite upon reaching moisture.
-
化学性质
強い還元性,水と激しく反応し,着火のおそれがある
-
使用用途
水素化物の中でも水素含有量が非常に高く、水素化リチウムは注目されています。水素化リチウムを使用すると、効率的に水素の貯蔵や輸送が容易になると期待されています。現在、水素の需要が高まっていますが、水素は低密度かつ極低温沸点であり、輸送に課題があるためです。
また水素化リチウムは、放射線の放射線遮蔽材として使用可能です。一般的な放射線遮蔽材には、コンクリート、水、ホウ素、鉛、鉄、グラファイト (黒鉛) 、、水素化チタンなどがあります。その中でも水素化リチウムはメリットのある素材です。小型原子炉などの開発では、危険な放射線を遮蔽する素材の一つとして検討されています。
-
安全性プロファイル
Poison by inhalation. A
severe eye, skin, and mucous membrane
irritant. Upon contact with moisture, lithium
hydroxide is formed. The LiOH formed is
very caustic and therefore highly toxic,
particularly to lungs and respiratory tract,
skin, and mucous membranes. The powder
ignttes spontaneously in air. The solid can
ignite spontaneously in moist air. Mixtures
of the powder with liquid oxygen are
explosive. Ignttes on contact with dinitrogen oxide, oxygen + moisture. To fight fire, use
special mixtures of dry chemical. See also
LITHIUM COMPOUNDS and
HYDRIDES.
-
職業ばく露
Lithium hydride is used in preparation
of lithium aluminum hydride; as a desiccant; it is used in
hydrogen generators and in organic synthesis as a reducing
agent and condensing agent with ketones and acid esters; it
is reportedly used in thermonuclear weapons.
-
貯蔵
The product should be handled under an inert atmosphere to avoid contamination and a fire. Powdered lithium hydride burns readily when exposed to the air. However, large pieces of the material are less flammable. Lithium hydride, like other strong bases, is harmful to the skin and should be handled with caution.
-
合成方法
水素と金属の直接反応でつくる
-
輸送方法
UN1414 Lithium, Hazard Class: 4.3; Labels:
4.3-Dangerous when wet material. UN2805 Lithium
hydride, fused solid, Hazard Class: 4.3; Labels: 4.3-
Dangerous when wet material
-
純化方法
It should be a white powder; otherwise replace it. It darkens rapidly on exposure to air and is decomposed by H2O to give H2 and LiOH, and reacts with lower alcohols. One gram in H2O liberates 2.8L of H2 (could be explosive). [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.]
-
不和合性
A Strong reducing agent. Incompatible
with oxidizers, halogenated hydrocarbons; acids can cause
fire and explosion. Reacts with water, forming caustic lithium hydroxide and flammable hydrogen gas; reaction may
cause ignition. May ignite spontaneously in moist air and
may reignite after fire is extinguished. Dangerous when
wet. Reacts with water to form hydrogen and lithium
hydroxide. Powdered form and liquid oxygen form an
explosive compound. Decomposes exothermically on contact with acids and upon heating to about 500�C, producing
flammable hydrogen gas. Reacts with carboxylic acids,
lower alcohols; chlorine, and ammonia (at 400�C), forming
explosive hydrogen gas.
-
廃棄物の処理
Lithium hydride may be
mixed with sand, sprayed with butanol and then with water,
neutralized and flushed to a sewer with water