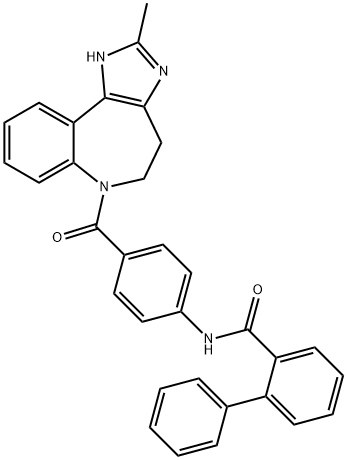
コニバプタン
化学名:コニバプタン
CAS番号.210101-16-9
英語名:CONIVAPTAN
CBNumberCB51011161
MFC32H26N4O2
MW498.583
MOL File210101-16-9.mol
别名
コニバプタン
コニバプタン 化学特性,用途語,生産方法
-
効能
利尿薬, バソプレシン受容体拮抗薬 -
説明
Conivaptan, a vasopressin antagonist, was discovered and developed by Yamanouchi for the treatment of hyponatraeum associated with congestive heart failure. -
定義
ChEBI: The amide resulting from the formal condensation of 4-[(biphenyl-2-ylcarbonyl)amino]benzoic acid with the benzazepine nitrogen of 2-methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine. It is an antagonist for two of the three types of argini e vasopressin (AVP) receptors, V1a and V2. It is used as its hydrochloride salt for the treatment of hyponatraemia (low blood sodium levels) caused by syndrome of inappropriate antidiuretic hormone (SIADH). -
合成
After looking at several different approaches to the synthesis, a convergent approach, shown in the Scheme 4, was developed for large scale synthesis. Bromination of benzazepinone 10 with pyridinium hydrobromide perbromide in chloroform followed by recrystallization gave bromide 11. Reaction of bromide 11 with ethaneimidate hydrochloride in the presence of potassium carbonate in toluene or chloroform gave the desired imidazole 12 in 69% yield. Although chlo-roform provided a slightly better yield, for large scale preparation, toluene was used to minimize halogenated solvent waste and because the quality of product was similar or better than with use of chloroform. Deprotection of the tosylate was found to be effective with heating the sulfonamide 12 in 80% sulfuric acid at 80oC. The benzazepinone product 13 was obtained in 90% yield after crystallization from acetonitrile and water mixture.
Synthesis of the coupling partner 16 required to provide conivaptan was synthesized in 95% yield from biphenyl 2- benzoic acid via sequential reaction with thionyl chloride in toluene followed by coupling with aminobenzoic acid in acetone with N,N-dimethylaniline as a base. High quality acid 16 was obtained by crystallization from DMF and water. The acid 16 was activated by converting it into acid chloride with thionyl chloride in acenonitrile, to which was added imidazo benzazepine 13 in toluene and, after recrystallization in acidic ethanol, gave conivaptan hydrochloride (III) in 90% yield.
コニバプタン 生産企業
Global(39)Suppliers
-
電話番号 0086-13720134139
電子メール candy@biochempartner.com
-
Hubei Jusheng Technology Co.,Ltd.
電話番号 18871490254
電子メール linda@hubeijusheng.com
-
電話番号 +86-0371-86658258<br/>+8613203830695
電子メール factory@coreychem.com
-
電話番号 +1-781-999-5354<br/>+1-00000000000
電子メール marketing@targetmol.com
-
電話番号 0592-5800732;<br/>+8613806035118
電子メール xie@china-sinoway.com
-
Wuhan Topule Biopharmaceutical Co., Ltd
電話番号 +8618327326525
電子メール masar@topule.com
-
電話番号 +86-852-30606658
電子メール market18@leapchem.com
-
Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
電話番号 +86-18791163155<br/>+86-13119157289
電子メール 13119157289@163.com
-
Wuhan Jingkang en Biomedical Technology Co., Ltd
電話番号 +8613720134139
電子メール orders@jknbiochem.com
-
Amadis Chemical Company Limited
電話番号 571-89925085
電子メール sales@amadischem.com
1of2