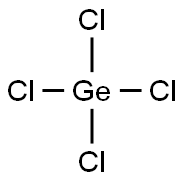ChemicalBook > CAS DataBase List > GERMANIUM(IV) CHLORIDE
GERMANIUM(IV) CHLORIDE
GERMANIUM(IV) CHLORIDE
- CAS No.10038-98-9
- Chemical Name:GERMANIUM(IV) CHLORIDE
- CBNumber:CB9852574
- Molecular Formula:Cl4Ge
- Formula Weight:214.45
- MOL File:10038-98-9.mol
GERMANIUM(IV) CHLORIDE Property
- Melting point -49.5 °C
- Boiling point 83 °C(lit.)
- Density 1.844 g/mL at 25 °C
- vapor pressure 76 mm Hg ( 20 °C)
- refractive index 1.4644
- Flash point 83.1°C
- storage temp. Store below +30°C.
- solubility Soluble in ether, benzene, chloroform, carbon tetrachloride. Insoluble in hydrochloric acid and sulphuric acid.
- form Liquid
- Specific Gravity 1.88
- color Colorless
- PH <1 (H2O, 20℃)
- Water Solubility DECOMPOSES
- Sensitive Moisture Sensitive
- Hydrolytic Sensitivity 8: reacts rapidly with moisture, water, protic solvents
- Merck 14,4410
- Dielectric constant 2.4(25℃)
- CAS DataBase Reference 10038-98-9(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII YSV1R803C0
- NIST Chemistry Reference Germanium tetrachloride(10038-98-9)
- EPA Substance Registry System Germane, tetrachloro- (10038-98-9)
- UNSPSC Code 12352302
- NACRES NA.22
Safety
- Hazard Codes :C
- Risk Statements :14-34-67-40-37
- Safety Statements :26-27-36-45-8-36/37/39-25
- RIDADR :UN 3390 6.1/PG 1
- WGK Germany :3
- RTECS :LY5225000
- F :10-19
- TSCA :Yes
- HS Code :2827 39 85
- HazardClass :8
- PackingGroup :II
-
NFPA 704:
0 3 1 W
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H290-H314-H335-H410
- Precautionary statements P234-P261-P273-P280-P303+P361+P353-P305+P351+P338
GERMANIUM(IV) CHLORIDE Price
More Price(5)
- Brand: Sigma-Aldrich(India)
- Product number: 8.04079
- Product name : Germanium(IV) chloride
- Purity: for synthesis
- Packaging: 25ML
- Price: ₹36370
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 208450
- Product name : Germanium(IV) chloride
- Purity: 99.99% trace metals basis
- Packaging: 25G
- Price: ₹27701.18
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 208450
- Product name : Germanium(IV) chloride
- Purity: 99.99% trace metals basis
- Packaging: 25G
- Price: ₹27701.18
- Updated: 2022/06/14
- Buy: Buy
- Brand: ottokemi
- Product number: G 1270
- Product name : Germanium(IV) chloride, 99.9999%
- Purity: 99.99%
- Packaging: 25gm
- Price: ₹49905
- Updated: 2022/05/26
- Buy: Buy
- Brand: ottokemi
- Product number: G 1270
- Product name : Germanium(IV) chloride, 99.9999%
- Purity: 99.99%
- Packaging: 100gm
- Price: ₹139059
- Updated: 2022/05/26
- Buy: Buy
GERMANIUM(IV) CHLORIDE Chemical Properties,Usage,Production
- Physical Properties Colorless liquid; density 1.879 g/cm3 at 20°C and 1.844 g/cm3 at 30°C; refractive index 1.464; solidifies at –49.5°C; decomposes in water; soluble in alcohol, ether, benzene, chloroform and carbon tetrachloride; insoluble in concentrated hydrochloric and sulfuric acids.
-
Preparation
Germanium(IV) chloride is prepared by reacting germanium metal with chlorine; or by treating germanium oxide, GeO2, with hydrochloric acid:
Ge + 2Cl2 → GeCl4
GeO2 + 4HCl → GeCl4 + 2H2O
Germanium(IV) chloride often is obtained as a byproduct of germanium metal production. The process involves heating germanium oxide, GeO2, with sodium chloride and coal. The vapors of germanium(IV) chloride and other volatile chlorides formed from the impurity metals are condensed. The product is isolated by fractional distillation. Further purification may be achieved by fractional distillation in 8N HCl and chlorine, or in the presence of other oxidizing agents in quartz stills.
Germanium(IV) chloride also is obtained by chlorination of germanium(II) chloride at ambient temperature. The reaction is rapid.
GeCl2 + Cl2 → GeCl4 -
Reactions
Germanium(IV) chloride reacts with water, hydrolyzing to germanium oxide and hydrochloric acid:
GeCl4 + 2H2O → GeO2 + 4HCl
The rate of hydrolysis is slower than the corresponding silicon analog, with hydrolysis occurring only partially. When heated with hydrogen at 1,000°C in a quartz reactor, it is converted into germanium(I) chloride, condensing onto the wall of the reactor:
2GeCl4 + 3H2→ 2GeCl + 6HCl
When vapors of GeCl4 are passed over germanium at elevated temperatures, the product is germanium(II) chloride, GeCl2:
GeCl4 + Ge→ 2GeCl2
Reaction with lithium aluminum hydride in ether forms monogermane, GeH4:
GeCl4 + LiAlH4 →GeH4 + LiCl + AlCl3
Reactions with antimony trifluoride, SbF3 in the presence of antimony pentachloride, SbCl5, form mixed halides of compositions: GeCl3F, GeCl3F2, GeCl2F2, and GeClF3.
Reactions with alcohols in the presence of an amine yield alkoxides:
GeCl4 + 4CH3OH + 4C2H5NH2 → Ge(OCH3)4 + 4C2H5N•HCl
Germanium forms six coordinate adducts, such as GeCl4(L)2 with many neutral ligands. - Chemical Properties Colorless liquid. decomposes in water. Insoluble in concentrated hydrochloric acid; soluble in carbon disulfide, chloroform, benzene, alcohol, and ether.
- Physical properties Colorless liquid; density 1.879 g/cm3 at 20°C and 1.844 g/cm3 at 30°C; refractive index 1.464; boils at 86.5°C; solidifies at -49.5°C; decomposes in water; soluble in alcohol, ether, benzene, chloroform and carbon tetrachloride; insoluble in concentrated hydrochloric and sulfuric acids.
- Uses It is used as a catalyst in the conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids, as a reducing agent in combination with triphenylphosphine (TPP) for the reduction of alpha-bromo carboxylic acid derivatives, and in the production of pure germanium. It also finds use as an intermediate for several optical processes. It is one of the most important dopants in silica glass for optical fibers. It is employed in the preparation of germanium dioxide which is used for wide camera lens, microscopy, IR-transparent glasses/windows/lenses, and for the core of fiber-optic lines.
-
Uses
Germanium(IV) chloride is used in the microwave preparation of Ge2Cl6, a colorless crystalline material which was included in a further study of low-valent germanium compounds.1
Germanium(IV) chloride is used in the preparation of many germanium compounds. - Hazard Toxic material
- Flammability and Explosibility Non flammable
- Safety Profile Poison by intravenous route. Mddly toxic by inhalation. A skin, severe eye, and mucous membrane irritant. Will react violently with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of Cl-. See also GERMANIUM COMPOUNDS.
- Purification Methods Traces of Cl2 and HCl can be removed from the liquid by blowing dry air through it for a few hours at room temperature or by shaking it with Hg or Hg2Cl2 and then fractionating it in a vacuum. It decomposes on heating at 950o. It has a sharp penetrating odour and fumes in moist air to give a chalky coat of GeO2. It is slowly hydrolysed by H2O to give GeO2, but distils from conc HCl. [Foster et al. Inorg Synth II 109 1946, Dennis & Hance J Am Chem Soc 44 304 1922, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 715 1963.] LACHRYMATORY. Glass powder (100-300 mesh). Washed with 10% HNO3, water and dry in air.
GERMANIUM(IV) CHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(119)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Antai Fine Chemical Technology Co.,Limited
- Tel: 18503026267
- Email:info@antaichem.com
- Country:CHINA
- ProdList:9636
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
CD Chemical Group Limited
- Tel: +8615986615575
- Email:info@codchem.com
- Country:China
- ProdList:20342
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8772
- Advantage:58
-
Supplier:
PT CHEM GROUP LIMITED
- Tel:+86-85511178;<br/>+86-85511178;
- Email:peter68@ptchemgroup.com
- Country:China
- ProdList:35425
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
- Tel: +8618950047208
- Email:ellena@amitychem.com
- Country:China
- ProdList:43416
- Advantage:58
Related articles
10038-98-9, GERMANIUM(IV) CHLORIDERelated Search:
- Sodium chloride Lithium chloride Magnesium chloride hexahydrate Choline chloride Barium chloride Ammonium chloride Aluminum chlorohydrate Zirconium tetrachloride Stannic chloride pentahydrate Ferrous chloride Tin tetrachloride Potassium chloride Tetrachlorosilane Calcium chloride (S)-tert-Butyl 6-amino-2-((tert-butoxycarbonyl)amino)hexanoate hydrochloride Calcium chloride hexahydrate Germanium Hydrochloric acid
- Metal and Ceramic Science
- Materials Science
- Germanium Salts
- Germanium
- Chemical Synthesis
- Catalysis and Inorganic Chemistry
- Inorganics
- metal halide
- Ultra-High Purity Materials
- Ge
- Salts
- 无机化工原料
- 大化工产品
- 金属催化剂
- 化工原料
- 金属有机物
- 无机盐
- 无机化工产品
- Ce液化物及氯酸盐
- Catalysis and Inorganic Chemistry
- Cl4Ge
- 10038-93-9
- 氯化锗 溶液
- 氯化锗(IV), 99.9999% (METALS BASIS)
- 氯化锗, 99.9999% (METALS BASIS)
- 氯化锗(Ⅳ)
- 四氯化鍺
- 氯化锗(IV)
- 四氯化锗
- 氯化锗
- 10038-98-9
- Germanium(IV) chloride, 95%
- Germanium(lV) chloride
- Germanium(IV) chloride for optical fibre
- ermanium(IV) chL
- Germanium(IV) chL
- GerManiuM tetrachloride, 99.99% 5GR
- GerManiuM tetrachloride, 99.99%, (trace Metal basis)
- GerManiuM tetrachloride, (trace Metal basis)
- Germanium tetrachloride, (trace metal basis), 99.99%
- Germanium chloride/ 99.999%
- Germanium(IV)chloride 99,9999%
- Germanium(IV) tetrachloride
- GERMANIUM CHLORIDE, 99.9999%
- geiTnanium tetrachloride
- Germanium (IV) chloride, 99% (metals basis)
- GERMANIUM TETRACHLORIDE 99.999%
- Germanium Tetrachloride (on conversion to germanium)
- Germanium(IV) chloride, 99.9999% (metals basis)
- GERMANIUM(IV) CHLORIDE: (99.9999%-GE) PURATREM, AMPOULED
- Germaniumtetrachlorid
- Germanium(IV)chloride(99.9999%-Ge)PURATREM
- Germanium(IV)chloride(99.99%-Ge)PURATREM
- GERMANIUM(+4)CHLORIDE
- GERMANIUM CHLORIDE
- GERMANIUM TETRACHLORIDE
- Germanium tetrachloride 99.9%
- GermaniumTetrachloride,99.99%