ChemicalBook > CAS DataBase List > PARAMETHADIONE (500 MG)
PARAMETHADIONE (500 MG)
PARAMETHADIONE (500 MG)
- CAS No.115-67-3
- Chemical Name:PARAMETHADIONE (500 MG)
- CBNumber:CB9500518
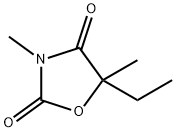
- Molecular Formula:C7H11NO3
- Formula Weight:157.17
- MOL File:115-67-3.mol
PARAMETHADIONE (500 MG) Property
- Melting point 31-32 °C
- Boiling point 281.82°C (rough estimate)
- Density d425 1.1180-1.1240
- refractive index nD25 1.449
- pka -2.18±0.40(Predicted)
- EWG's Food Scores 1
- FDA UNII Z615FRW64N
- Proposition 65 List Paramethadione
- ATC code N03AC01
- EPA Substance Registry System Paramethadione (115-67-3)
Safety
- Hazardous Substances Data :115-67-3(Hazardous Substances Data)
- Toxicity :LD50 orl-mus: 1000 mg/kg 27ZQAG -,280,72
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
PARAMETHADIONE (500 MG) Chemical Properties,Usage,Production
- Originator Paradione,Abbott, US ,1949
- Uses Paramethadione is also used in minor forms of epilepsy.
- Uses Paramethadione acts as an antiepileptic and anticonvulsant agent.
- Definition ChEBI: Paramethadione is an oxazolidinone.
-
Manufacturing Process
About 143.1 grams (one mol) of 5-methyl-5-ethyloxazolidine-2,4-dione is dissolved in 300 cc of methanol containing 23 grams of sodium. To the above mixture is added 126 grams of dimethyl sulfate in 10 cc portions while the temperature is maintained at about 50°C by external cooling. The mixture is then heated briefly to boiling, cooled, diluted with about 500 cc of water and extracted with two 250 cc portions of benzene. The benzene extract is separated, washed once with sodium bicarbonate solution and once with water. The benzene is removed by evaporation on a steam bath and the residue is fractionally distilled. The material boiling at 112° to 116°C at 25 mm pressure is taken; nD25=1.4495. Upon further fractionation, a very pure specimen boils at 101°-102°C at 11 mm.
The 5-methyl-5-ethyloxazolidine-2,4-dionemay be prepared by reacting methyl ethyl ketone with sodium cyanide and with ammonium thiocyanate followed by desulfurization. This intermediate may also be prepared by condensing α-hydroxy-α-methylbutyramide with ethyl chlorocarbonate or by condensing ethyl α-hydroxy-α-methylbutyrate with urea. Another method described (Traube and Aschar, Ber., 46, 2077-1913) consists in the condensation of ethyl α-hydroxy-α-methylbutyrate with guanidine followed by hydrolysis. - brand name Paradione (Abbott).
- Therapeutic Function Anticonvulsant
- Safety Profile Moderately toxic by ingestion and intraperitoneal routes. Experimental teratogenic effects. Other experimental reproductive effects. Whenheated to decomposition it emits toxic fumes of NOx.
- Synthesis Paramethadione, 5-ethyl-3,5-dimethyloxazolidine-2,4-dione (9.8.3), differs from trimethadione only in the substitution of one methyl group with an ethyl group. It is synthesized in a completely analogous manner, except that it comes from 2-hydroxy-2-methylbutyric acid instead of 2-hydroxyisobutyric acid [29].
PARAMETHADIONE (500 MG) Preparation Products And Raw materials
Raw materials
Preparation Products
Global(26)Suppliers
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Dideu Industries Group Limited
- Tel:+86-29-89586680<br/>+86-15129568250
- Email:1026@dideu.com
- Country:China
- ProdList:22854
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: Sinopharm Chemical Reagent Co,Ltd.
- Tel:86-21-63210123
- Email:sj_scrc@sinopharm.com
- Country:China
- ProdList:9815
- Advantage:79
- Supplier: Beijing HuaMeiHuLiBiological Chemical
- Tel:010-56205725
- Email:waley188@sohu.com
- Country:China
- ProdList:12335
- Advantage:58
- Supplier: Shaanxi Dideu Medichem Co. Ltd
- Tel:029-029-81124267<br/>15229202216
- Email:1073@dideu.com
- Country:China
- ProdList:10005
- Advantage:58
- Supplier: Shaanxi DIDU pharmaceutical and Chemical Co., Ltd
- Tel:029-029-81148696<br/>18161915376
- Email:1040@dideu.com
- Country:China
- ProdList:9799
- Advantage:58
- Supplier: Biosynth Biological Technology (Suzhou) Co Ltd
- Tel: 51288865780
- Email:sales@biosynth.com
- Country:China
- ProdList:233051
- Advantage:58
115-67-3, PARAMETHADIONE (500 MG)Related Search:
- 化合物 T33882
- 甲乙双酮
- 115-67-3
- Nicotinamide Impurity 215
- Paramethadionum
- HSDB 3245
- BRN 0127715
- Paramethadione (1497000)
- PARAMETHADIONE (500 MG) USP/EP/BP
- PARAMETHADIONE (500 MG)
- Isoethadione
- a348
- A 348
- 5-ethyl-3,5-dimethyloxazolidine-2.4-dione
- 5-Ethyl-3,5-dimethyloxazolidine-2,4-dione
- 5-ethyl-3,5-dimethyl-4-oxazolidinedione
- 5-Ethyl-3,5-dimethyl-2,4-oxazolidinedione
- 5-Ethyl-3,5-dimethyl-1,3-oxazolidine-2,4-dione
- 3,5-dimethyl-5-ethyloxazolidine-2,4-dione
- 2,4-Oxazolidinedione, 5-ethyl-3,5-dimethyl-
- Paramethodione
- Paramethadione
- Parametadione
- Paradione
- 5-ethyl-3,5-dimethyl-oxazolidine-2,4-quinone
- Isethadionum