ChemicalBook > CAS DataBase List > Ammonium carbonate
Ammonium carbonate
Outline Solubility in water (g/100ml) Related reactions of the formula Toxcity Chemical properties Uses Production method Category Toxicity grading Acute toxicity Flammability hazard characteristics Storage characteristics Extinguishing agent
Ammonium carbonate
- CAS No.506-87-6
- Chemical Name:Ammonium carbonate
- CBNumber:CB9393644
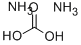
- Molecular Formula:CH8N2O3
- Formula Weight:96.09
- MOL File:506-87-6.mol
Ammonium carbonate Property
- Melting point 58 °C
- Boiling point 179.58°C (rough estimate)
- Density 1.5
- vapor density 2.7 (vs air)
- vapor pressure 760 mm Hg @ 600C
- refractive index 1.4616 (estimate)
- storage temp. Store at RT.
- solubility H2O: 0.1 g/mL at 25 °C, clear, colorless
- form Solid
- color White to yellow
- Odor strong odor of NH3
- PH Range 9
- Water Solubility SOLUBLE
- Sensitive Hygroscopic
- Merck 14,508
- BRN 3627235
- LogP -0.809 (est)
- CAS DataBase Reference 506-87-6(CAS DataBase Reference)
- FDA 21 CFR 184.1137; 582.1137
- EWG's Food Scores 1
- FDA UNII PDP691CN28
- EPA Substance Registry System Ammonium carbonate (506-87-6)
- UNSPSC Code 12352301
- NACRES NA.55
Safety
- Hazard Codes :Xn
- Risk Statements :22-52/53
- Safety Statements :24/25
- RIDADR :UN 9084
- WGK Germany :1
- RTECS :BP1925000
- F :13
- TSCA :Yes
- HS Code :28369940
- Hazardous Substances Data :506-87-6(Hazardous Substances Data)
- Toxicity :LD50 ivn-mus: 96 mg/kg AJVRAH 29,897,68
-
NFPA 704:
0 2 1
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P301+P312+P330
Ammonium carbonate Price
More Price(23)
- Brand: Sigma-Aldrich
- Product number: 207861
- Product name : Ammonium carbonate
- Purity: ACS reagent, ≥30.0% NH3 basis
- Packaging: 25G
- Price: ₹2662.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 207861
- Product name : Ammonium carbonate
- Purity: ACS reagent, ≥30.0% NH3 basis
- Packaging: 25G
- Price: ₹2662.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 207861
- Product name : Ammonium carbonate
- Purity: ACS reagent, ≥30.0% NH3 basis
- Packaging: 100G
- Price: ₹5423.33
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 207861
- Product name : Ammonium carbonate
- Purity: ACS reagent, ≥30.0% NH3 basis
- Packaging: 100G
- Price: ₹5423.33
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 379999
- Product name : Ammonium carbonate
- Purity: 99.999% trace metals basis
- Packaging: 10G
- Price: ₹5564.05
- Updated: 2022/06/14
- Buy: Buy
Ammonium carbonate Chemical Properties,Usage,Production
- Outline Ammonium carbonate is positive ammonium salt of carbonic acid normal salt, the formula is (NH4)2CO3. Pure product is colorless or white cubic crystal or powder, it has strong smell of ammonia. Industrial product is complex salt of ammonium bicarbonate and ammonium carbamate, it is white, flaky or small block of solid product crushed form. It is often with a molecular crystal water, it is hygroscopic, soluble in water, it can decompose in case of hot water. It is insoluble in ethanol and carbon disulfide. Ammonium carbonate can rapidly decompose into ammonia, carbon dioxide and water at 58℃. Ammonium carbonate can be obtained by ammonia introduces into solution of sodium carbonate with half times, the solution crystallizes at 30°C. It gradually loses ammonia to form ammonium bicarbonate in air.
- Solubility in water (g/100ml) The grams which dissolve per 100 ml of water:100g/20 ℃.
-
Related reactions of the formula
At room temperature for significant decomposition: (NH4) 2CO3 → 2NH3 + CO2 + H2O
At low temperature and a certain pressure, carbon dioxide and water with an excess of ammonia, ammonium carbonate can be obtained: 2NH3 + CO2 + H2O → (NH4) 2CO3
Ammonium sulfate and calcium carbonate suspension under heating to generate ammonium carbonate: (NH4) 2SO4 + CaCO3 → (NH4) 2CO3 + CaSO4
Urea in aqueous solution will gradually react with water to form ammonium carbonate: CO (NH2) 2 + 2H2O → (NH4) 2CO3 - Toxcity If it splashes into the eye accidentally, rinse immediately with plenty of water. It has stimulating effect on the skin. It should pay attention to dust prevention and dust extraction, respiratory protection, skin protection.
- Chemical properties It is matte orthorhombic crystalline powder. It has strong ammonia odor. It usually can not get anhydrous salt, industrial salt is actually a complex of ammonium bicarbonate and ammonium carbamate. The amount of ammonia is 31%, The amount of carbon dioxide is 56%. It is soluble in water, insoluble in ethanol, carbon disulfide, and concentrated ammonia. It is unstable in the air, it will gradually become ammonium bicarbonate and ammonium carbamate. When be dried at 58℃, it can easily decompose, release ammonia and carbon dioxide. Aqueous solution begins to decompose at 70℃. It is unstable for light and heat. It has slightly hygroscopic.
-
Uses
It is used as raw material for baking powder, various ammonium salts, buffer agent, auxiliaries, fertilizer and analytical reagent. Edible ammonium carbonate is used as buffer, neutralizing agent, leavening agent, fermentation promoter (manufacture of wine).
It is used for fire fighting, detergents, and used in medicine, rubber, and other industrial fermentation.
The above information is edited by the chemicalbook of Wang Xiaodong. -
Production method
Carbonization method: Carbon dioxide, ammonia and steam synthesized directly sodium carbonate, it passes through the cooling chamber, uses water to direct cooling, and then it is refined to obtain ammonium carbonate products.
2NH3 + CO2 + H2O → (NH4) 2CO3 - Category Toxic substances.
- Toxicity grading highly toxic.
- Acute toxicity Intravenous-Mouse LD50: 96 mg/kg; Intravenous-Dogs LDL0: 200 mg/kg.
- Flammability hazard characteristics It can produce toxic fumes of nitrogen oxides and ammonia at high temperature.
- Storage characteristics Treasury ventilation low-temperature drying.
- Extinguishing agent Dry powder, foam, sand, carbon dioxide, water mist.
- Chemical Properties Anunonium carbonate is a white water-soluble, volatile solid prepared by reaction of NH4OH and CO2 and crystallizing from dilute alcohol. Ammonium carbonate loses NH3,CO2, and H20 at ordinary temperatures, and rapidly at 58°C.
- Chemical Properties Ammonium carbonate is a colorless crystal or white lumpy powder with a strong ammonia odor. The odor specific gravity (gas)52.7; Threshold is ,5 ppm as ammonia gas.
- Physical properties Colorless or translucent hard crystalline mass or white cubic crystals or powder; sharp taste; odor of ammonia; decomposes at 58°C; slow decomposition at ambient temperatures; readily dissolves in cold water; decomposes in hot water; insoluble in liquid ammonia, alcohol and carbon disulfide.
- Uses Pharmaceutic aid (source of ammonia).
- Uses Ammonium carbonate is widely utilized as a leavening agent in lebkuchen, cookies and flat biscuits. It finds an important application as an emetic and an active ingredient in some cough syrups. It is the main component in smelling salts and an active ingredient in some smokeless tobacco products, shampoos and dyes used in textile industries. It is an important predecessor to the modern leavening agent?s baking soda and baking powder. It serves as a foaming agent for the production of expanded material. It is also used as a reagent for analytical purposes in the chemical industry.
- Uses Ammonium Carbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. it is prepared by the sublima- tion of a mixture of ammonium sulfate and calcium carbonate, and occurs as a white powder or a hard, white translucent mass.
- Definition A mixture of ammonium acid carbonate and ammo- nium carbamate.
- Preparation Ammonium carbonate is obtained by passing carbon dioxide into aqueous ammonia solution in a column or tower. Ammonia, carbon dioxide and water vapor are distilled and the vapors condensed into a solid crystalline mass. It also may be prepared by subliming a mixture of ammonium sulfate and calcium carbonate.
- Definition ammonium carbonate: A colourlessor white crystalline solid,(NH4)2CO3, usually encountered asthe monohydrate. It is very soluble incold water. The compound decomposesslowly to give ammonia, water,and carbon dioxide. Commercial ‘ammoniumcarbonate’ is a double saltof ammonium hydrogencarbonateand ammonium aminomethanoate(carbamate), NH4HCO3.NH2COONH4.This material is manufactured byheating a mixture of ammoniumchloride and calcium carbonate andrecovering the product as a sublimedsolid. It readily releases ammoniaand is the basis of sal volatile. It isalso used in dyeing and wool preparationand in baking powders.
- General Description A colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.
- Air & Water Reactions Water soluble.
- Reactivity Profile Ammonium carbonate decomposes when heated to give gaseous ammonia and gaseous carbon dioxide. Reaction is non-explosvie. Causes decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
- Hazard Evolves irritating fumes when heated.
- Health Hazard Inhalation causes irritation of nose and throat. Ingestion may cause gastric irritation. Contact with eyes or skin causes irritation.
- Agricultural Uses Ammonium carbonate, (NH4)2CO3, is an intermediate product formed during the synthesis of urea. Ammonium carbonate on decomposition yields urea and water.
- Safety Profile Poison by subcutaneous and intravenous routes. When heated to decomposition it emits toxic fumes of NO, and NH3.
- Potential Exposure It is used in dyeing, tanning, medicines, fire extinguishers; to make casein glue; ammonia salts; and baking powders. A laboratory reagent.
- Shipping UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
- Incompatibilities Acids, acid salts; salts of iron and zinc, alkaloids, calomel and tartar emetic. Keep cool, below 38 C. Contact with inorganic acids may form CO2, heat, and dangerous spattering.
- Waste Disposal Slowly deposit in a large container of water. Add excess amounts of soda ash and let stand for 24 hours. Decant to another container, neutralize with hydrochloric acid, and drain with an excess of water. Ship to landfill.
Ammonium carbonate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(419)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883<br/>+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21629
- Advantage:55
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29860
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hebei Jimi Trading Co., Ltd.
- Tel: +86 319 5273535
- Email:bestoneforyou@sina.com
- Country:CHINA
- ProdList:287
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Shandong chuangyingchemical Co., Ltd.
- Tel:18853181302
- Email:sale@chuangyingchem.com
- Country:CHINA
- ProdList:5906
- Advantage:58
506-87-6, Ammonium carbonateRelated Search:
- Sodium carbonate Lithium carbonate Sodium chloride Ethylene carbonate Calcium carbonate Dimethyl carbonate Guanidine carbonate Calcium carbide Barium carbonate Boron carbide 8-CHLOROADENOSINE-3',5'-CYCLIC MONOPHOSPHOROTHIOATE, RP-ISOMER SODIUM SALT Strontium carbonate Carbonyl iron powder Zirconium carbonate oxide Vanadium carbides Zirconium carbide Titanium carbide Sodium bicarbonate
- Industrial/Fine Chemicals
- Inorganics
- Salts
- Metal and Ceramic Science
- Materials Science
- Ammonium Salts
- 精细化工原料
- 其他化工产品
- 高端化学
- 有机类
- 固体类
- 大化工产品
- 无机产品
- 有机产品
- 一类
- 原料药
- 精细化工品
- 通用生化试剂-无机盐
- 无机酸类
- 添加剂
- 无机化工原料
- 化工原料
- 碳酸铵
- 化工
- 生化试剂
- 化工产品-无机化工
- 无机碱
- 有机铝化合物
- 酸
- 专业试剂
- 无机化合物和盐
- 合成试剂
- 通用试剂
- 其他无机物
- 无机盐
- 无机化工
- 无机化工产品
- Cd硝酸盐
- Inorganic Bases
- Synthetic Reagents
- NH42CO3
- C2H10N2O8Zr
- C2H11N3O5
- CH8O3N2
- NH42CO3H2O
- ApproxNH42CO3
- NH4HCO3NH2COONH4
- 碳酸铵饱和溶液
- 碳酸铵99.99%
- 碳酸铵,99%
- 碳酸铵缓冲溶液
- 碳酸铵 AMMONIUM CARBONATE
- AMMONIUM CARBONATE PURISS., MEETS ANALYTICAL SPECIFICATION OF NF, PH.聽FRANC., FCC
- 碳酸氨
- 碳酸铵试液(药典)
- 英国药典 碳酸铵溶液
- 欧洲药典 碳酸铵溶液
- 印度药典 碳酸铵溶液