ChemicalBook > CAS DataBase List > Tenofovir Alafenamide
Tenofovir Alafenamide
Tenofovir Alafenamide
- CAS No.379270-37-8
- Chemical Name:Tenofovir Alafenamide
- CBNumber:CB92627921
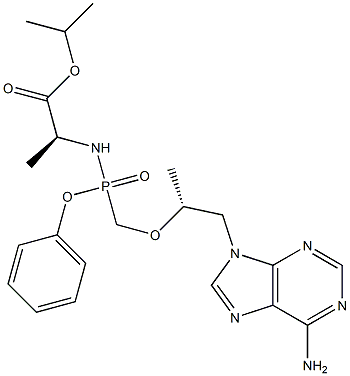
- Molecular Formula:C21H29N6O5P
- Formula Weight:476.47
- MOL File:379270-37-8.mol
Tenofovir Alafenamide Property
- Melting point >119°C (dec.)
- Boiling point 640.4±65.0 °C(Predicted)
- Density 1.39±0.1 g/cm3(Predicted)
- storage temp. 2-8°C(protect from light)
- solubility Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly)
- pka 4.21±0.10(Predicted)
- form Solid
- color White to Off-White
- Stability Hygroscopic
- InChIKey LDEKQSIMHVQZJK-QTJFZWIYNA-N
- SMILES C(OC(C)C)(=O)[C@H](C)NP(CO[C@H](C)CN1C2=C(N=C1)C(N)=NC=N2)(OC1=CC=CC=C1)=O |&1:6,12,r|
- FDA UNII EL9943AG5J
- NCI Drug Dictionary tenofovir alafenamide
- ATC code J05AF13
- UNSPSC Code 51111800
- NACRES NA.77
Safety
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H315-H318-H335
- Precautionary statements P261-P264-P271-P280-P302+P352-P304+P340-P305+P351+P338-P310-P321-P362+P364-P332+P313-P403+P233-P405-P501
Tenofovir Alafenamide Chemical Properties,Usage,Production
- Description Tenofovir Alafenamide (GS-7340) is a prodrug of tenofovir, which is a reverse transcriptase inhibitor, used to treat HIV and Hepatitis B.-Reverse Transcriptase inhibitor. It was developed by Gilead Sciences. Compared to tenofovir disoproxil fumarate, tenofovir alafenamide has a greater antiviral activity and better distribution into lymphoid tissues.
- Description Tenofovir alafenamide fumarate is an oral phosphonoamidate prodrug of the reverse transcriptase inhibitor tenofovir. It was approved by the USFDA for the treatment of chronic hepatitis B virus infection with compensated liver disease. Tenofovir alafenamide fumarate was discovered and developed by Gilead as a potentially safer form of the previously approved tenofovir disoproxil fumarate (Viread).
- Uses Tenofovir Alafenamide, also known as Tenofovir Impurity 51, is a prodrug of Tenofovir (T018500), which is a reverse transcriptase inhibitor used to treat HIV and Hepatitis B.
- Definition ChEBI: An L-alanine derivative that is isopropyl L-alaninate in which one of the amino hydrogens is replaced by an (S)-({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)( henoxy)phosphoryl group. A prodrug for tenofovir, it is used (as the fumarate salt) in combination therapy for the treatment of HIV-1 infection.
-
Synthesis
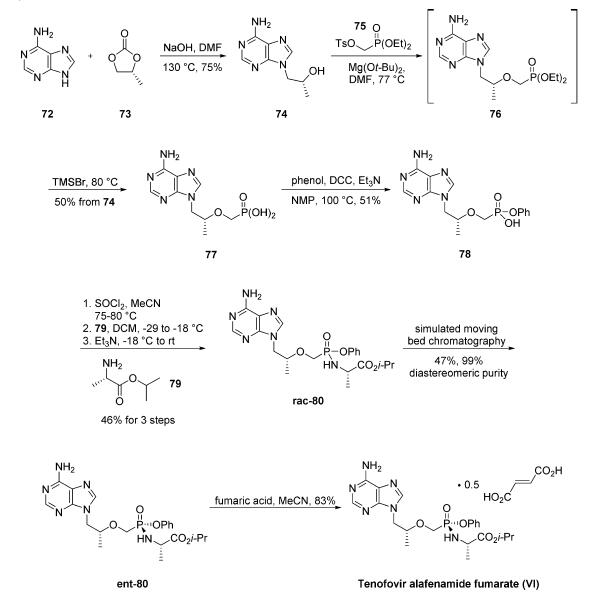
A multikilogram synthesis of tenofovir alafenamide fumarate
was described in a Gilead patent. Additional process
improvements on specific steps of the Gilead process have
been reported on 100 g scale, and these will be noted
throughout the description of the synthesis. The synthesis was
initiated with the alkylation of adenine (72) with (R)-propylene
carbonate (73) to give hydroxypropyl adenine 74 in 75% yield. It should be noted that sodium hydroxide can be
replaced by potassium bases with increased yields on 100 g
scale.27 Alkylation of 74 with diethyl p-toluenesulfonyloxymethylphosphonate
(75) gave intermediate 76, which was not
isolated. Hydrolysis of the phosphonate esters with trimethylsilyl
bromide followed by recrystallization from water gave
phosphonic acid 77 in 50% yield. Interestingly, replacing
Mg(Ot-Bu)2 with PhMgCl/t-BuOH led to improved yields for
the alkylation step (74 ?ú 76) on a 100 g scale. Additionally,
the authors note that conditions for hydrolyzing the
phosphonate ester can be modified using HCl or HBr for
improved yields on smaller scale. Dicyclohexylcarbodiimide
(DCC) coupling of 77 with phenol produced phosphonate 78
in 51% yield. This step was also reported to proceed in higher
yield on smaller scale by changing the solvent to cyclopentylmethyl
ether. Monophosphonate ester 78 was treated
with thionyl chloride followed by L-alanine isopropyl ester (79)
and triethylamine to give tenofovir alafenamide rac-80 as a
mixture of phosphonate diastereomers in 47% yield. The
diastereomers were separated using simulated moving bed
chromatography to give the desired diastereomer ent-80 in
47% yield and 99% diastereomeric purity. The diastereomers
could also be separated using a crystallization-induced dynamic
resolution of rac-80. Tenofovir alafenamide fumarate (VI)
was prepared from ent-80 and fumaric acid in 83% yield.
Tenofovir Alafenamide Preparation Products And Raw materials
Raw materials
Preparation Products
Global(373)Suppliers
-
Supplier:
Guangzhou Tosun Pharmaceutical Limited
- Tel: +8618922120635
- Email:sales@toref-standards.com
- Country:China
- ProdList:998
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Cangzhou Kangrui Pharma Tech Co. Ltd.,
- Tel:+86-18632776803<br/>+86-13833998158
- Email:cangzhoukangrui@126.com
- Country:China
- ProdList:739
- Advantage:58
-
Supplier:
Moxin Chemicals
- Tel: +8617320513646
- Email:Anna@molcoo.com
- Country:China
- ProdList:9684
- Advantage:58
-
Supplier:
Guangzhou Tosun Pharmaceutical Ltd
- Tel:+86-020-61855200-902<br/>+8618124244216
- Email:info@upharm.cn
- Country:China
- ProdList:897
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Nanjing Gold Pharmaceutical Technology Co. Ltd.
- Tel:025-84209270 15906146951
- Email:
- Country:CHINA
- ProdList:115
- Advantage:55
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
379270-37-8, Tenofovir AlafenamideRelated Search:
- Thionyl chloride D-Alanine Isopropyl Ester HCl 6N-HydroxyMethyl Tenofovir Disoproxil Tenofovir Related Compound 14 Tenofovir Alafenamide PMPA Impurity Pimecrolimus 9-[(R)-2-(Phosphonomethoxy)propyl]adenine monohydrate Tenofovir disoproxil 9-Propenyladenine Tenofovir DNA, SINGLE STRANDED, IMM. ON CELLULOSE, FROM CALF THYMUS* diisopropyl 2,2'-((((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)phosphoryl)bis(azanediyl))(2S,2'S)-dipropionate compound with methane (1:1) Tenofovir Related Compound 6 Tenofovir disoproxil fumarate phenyl hydrogen ((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)phosphonate Tenofovir alafenamide hemifumarate GS-7340 (fuMarate) Tenofovir Alafenamide Impurity 30
- 379270-37-8
- Intermediate
- Inhibitors
- 化工中间体-化学试剂
- 药靶配体
- 医药原料药API
- 柏锦生物
- 杂质对照品
- 常规产品
- 化工原料
- 医用原料
- 对照品-杂质对照品
- 医药化工类
- 医药原料
- 中间体
- 小分子抑制剂
- 原料药
- 抑制剂
- 小分子抑制剂,天然产物
- 医药原料药
- C25H33N6O9P
- C21H29N6O5P
- 1292275-56-7
- 134678-17-7
- 392275-56-7
- 79270-37-8
- 替诺福韦艾拉酚胺,10 MM DMSO 溶液
- 替诺福韦拉酚氨酯杂质21(替诺福韦艾拉酚胺杂质)对照品(FREE BASE)
- 磷丙替诺福韦(富马酸替诺福韦艾拉酚胺)
- 泰诺福韦艾拉酚胺杂质
- 替诺福韦艾拉酚胺(TAF BASE)
- 替诺福韦和阿拉芬胺
- 富马酸丙酚替诺福韦对照品
- 富马酸替诺福韦艾拉酚胺TAF
- L-丙氨酸,N-[(S)-[[(1R)-2-(6-氨基-9H-嘌呤-9基)-1-甲基乙氧基]甲基]苯氧基氧膦基],1-甲基乙基酯
- 替诺福韦艾拉酚胺(TFA)
- 富马酸替诺福韦甲酰胺
- 丙酚替诺福韦
- (S)-2-(((S)-((((R)-1-(6-氨基-9H-嘌呤-9-基)丙-2-基)氧基)甲基)(苯氧基)磷酰基)氨基)丙酸异丙酯
- 替诺福韦-阿拉芬酰胺-D6
- 替诺福韦艾拉酚胺(GS-7340)
- 替诺福韦丙氨苯丙酰胺
- 替诺福韦阿拉芬酰胺
- 替诺福韦-丙芬酰胺
- 泰诺福韦艾拉酚胺游离碱
- (S)-丙胺酸异丙酯2-{(S)-[((R)-1-(6-氨基-9H-嘌呤-9-基)-1-甲基乙氧基]甲基}-苯氧基磷酰基
- 替诺福韦艾拉酚胺原料药及其全线产品以及工艺
- 泰诺福韦艾拉酚胺(GS-7340)
- 泰诺福韦富马酸酯
- 磷丙替诺福韦
- 替诺福韦杂质51
- 替诺福韦艾拉酚胺中间体2
- N-[(S)-[[(1R)-2-(6-氨基-9H-嘌呤-9-基)-1-甲基乙氧基]甲基]膦酸酯基]-L-丙氨酸 1-甲基乙基酯
- 替诺福韦阿拉芬胺
- 替诺福韦艾拉酚胺富马酸盐 1392275-56-7
- 替诺福韦艾拉酚胺 250MG
- 磷丙替诺福韦(别名:GS7340)
- 富马酸替诺福韦艾拉酚胺