ChemicalBook > CAS DataBase List > Voriconazole-d3
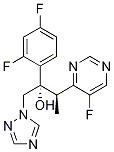
Voriconazole-d3
Voriconazole-d3
- CAS No.188416-29-7
- Chemical Name:Voriconazole-d3
- CBNumber:CB92610550
- Molecular Formula:C16H14F3N5O
- Formula Weight:349.31
- MOL File:188416-29-7.mol
Voriconazole-d3 Property
- Boiling point 508.6±60.0 °C(Predicted)
- Density 1.42±0.1 g/cm3(Predicted)
- pka 11.54±0.29(Predicted)
- form Solid
- color White to off-white
- FDA UNII USG4B1CD29
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501
Voriconazole-d3 Chemical Properties,Usage,Production
- Description Voriconazole-d3 is an Ergosterol biosynthesis inhibitor and a labeled form of Voriconazole. This mono triazole antifungal agent inhibits the growth of Candida, Cryptococcus, and Aspergillus species. Studies suggest that Voriconazole-d3 can be synthesized by modifying the structure of fluconazole. This antifungal agent functions by inhibiting cytochrome P450 dependent 14-α-sterol demethylase, which is responsible for the biosynthesis of Ergosterol. Furthermore, Voriconazole-d3 inhibits fungal growth, cell wall thinning, and cell membrane degradation.
- Uses Voriconazole is a triazole antifungal agent used primarily in the treatment or prevention of aspergillosis and candidal infections. However, its therapy may cause transient, asymptomatic serum aminotransferase elevations, and it is also a well-known cause of acute drug-induced liver injury.
- Application Voriconazole-d3 is derived from 2,4-Difluoro-α-(1H-1,2,4-triazolyl)acetophenone, which is an antifungal activity, particularly toward Candida albicans and Candida parapsilosis.
-
References
1. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species DOI:10.1128/AAC.41.8.1840
Belanger, P., et al. 1997. Antimicrob. Agents Chemother. 41: 1840-1842. PMID: 9257776
2. In vitro activities of voriconazole (UK-109,496) and four other antifungal agents against 394 clinical isolates of Candida spp
Marco, F., et al. 1998. Antimicrob. Agents Chemother. 42: 161-163. PMID: 9449278
3. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens
Espinel-Ingroff, A. 1998. J. Clin. Microbiol. 36: 198-202. PMID: 9431946
Voriconazole-d3 Preparation Products And Raw materials
Raw materials
Preparation Products
Global(76)Suppliers
-
Supplier:
HAINAN POLY PHARMCEUTICAL CO. LTD
- Tel:+86-0571-89385087<br/>+8613616530509
- Email:ff@hnpoly.com
- Country:China
- ProdList:118
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19805
- Advantage:58
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Jinan Shengqi pharmaceutical Co,Ltd
- Tel:86+18663751872
- Email:christine@shengqipharm.com
- Country:CHINA
- ProdList:491
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
HubeiwidelychemicaltechnologyCo.,Ltd
- Tel:18627774460
- Email:faith@widelychemical.com
- Country:CHINA
- ProdList:742
- Advantage:58
-
Supplier:
Shanghai Yingrui Biopharma Co.,Ltd
- Tel:21-33585366
- Email:export01@shyrchem.com
- Country:CHINA
- ProdList:1319
- Advantage:58
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12186
- Advantage:58
188416-29-7, Voriconazole-d3Related Search:
- 4-Ethyl-5-fluoropyrimidine Voriconazole Impurity 16 Ethanone, 1-(3,5-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)- 1-Butanesulfonic acid, 4-hydroxy-, methyl ester Pyrimidine, 4,4'-(1,2-dimethyl-1,2-ethanediyl)bis[2,6-dichloro-5-fluoro- (9CI) Pyrimidine, 4-bromo-6-(1-bromoethyl)-5-fluoro- 1,2,4-Triazole Voriconazole Impurity 53 Voriconazole (2R,3S/2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol ent-Voriconazole FENOFIBRIC ACID ETHYL ESTER (2R,3S/2S,3R)-3-(4-Chloro-5-fluoro-6-pyrimidinyl)-2-(2,4-difluorophenyl)butan-2-ol hydrochloride 4-Ethyl-5-fluoro-6-hydroxypyrimidine 6-(1-Bromoethyl)-4-chloro-5-fluoropyrimidine 2-Chloro-2',4'-difluoroacetophenone
- 主打产品
- 化学试剂
- 系列1
- 伏立康唑中间体
- (2R,3S/2S,3R)-2-(2,4-DIFLUOROPHENYL)-3-(5-FLUOROPYRIMIDIN-4-基)-1-(1H-1,2,4-TRIAZOL-1-YL)-2-BUTANOL
- (2R,3S/2S,3R)-2-(2,4-二氟苯基)-3-(5-氟嘧啶-4-基)-1-(1H-1,2,4-三唑-
- 伏立康唑EP杂质D、伏立康唑右旋体
- 化合物 (±)-VORICONAZOLE
- (2R,3S/2S,3R) -2-(2,4-二氟苯基)-3-(5-氟-4-嘧啶基) -1-(1H-1,2,4-三唑-1-基) -丁-2醇
- ()-伏立康唑-D3
- (2S,3R)-REL-2-(2,4-二氟苯基)-3-(5-氟嘧啶-4-基)-1-(1H-1,2,4-三唑-1-基)丁-2-醇
- (R,S)-Α-(2,4-二氟苯基)-5-氟-Β-甲基-Α-(1H-1,2,4-三唑-1-甲基)-4-嘧啶乙醇
- (R*,S*)-Α-(2,4-二氟苯基)-5-氟-Β-甲基-Α-(1H-1,2,4-三唑-1-甲基)-4-嘧啶乙醇
- 4-三唑-1-基甲基)-4-嘧啶乙醇
- 4-二氟苯基)-5-氟-Β-甲基-Α-(1H-1
- (R*,S*)3-(5-氟嘧啶-4-基)-2-(2,4-二氟苯基)-1-(1H-1,2,4-三唑-1-基)-2-丁醇
- (R*,S*)-Α-(2,4-二氟苯基)-5-氟-Β-甲基-Α-(1H-1,2,4-三唑-1-基甲基)-4-嘧啶乙醇
- 伏立康唑中间体II
- 188416-29-7
- (±)-Voriconazole
- (2RS,3SR)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
- Fluconazole Intermediate 2
- Intermediate 2 of voriconazole
- Voriconazole Intermediate II
- VORICONAZOLE-D3
- (2R, 3S/2S, 3R)-3-(5-Fluoropyrimidin-4-yl)-2-(2,4-difluorophyenyl)-1-(1H-1,2,4 triazol-1-yl) butan-2-ol {Racemic Voriconazole}
- 4-Pyrimidineethanol, α-(2,4-difluorophenyl)-5-fluoro-β-methyl-α-(1H-1,2,4-triazol-1-ylmethyl)-, (αR,βS)-rel-
- (2R,3S/2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoro-4-pyriMidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (RaceMic Voriconazole)ChemicalBook
- 4-triazol-1-yl)-2-butanol (RaceMic Voriconazole)
- 4-Difluorophenyl)-3-(5-fluoro-4-pyriMidinyl)-1-(1H-1
- 3R)-2-(2
- (2S,3R)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol
- (R *, S *) 3- (5-fluoropyrimidin-4-yl) -2- (2,4-difluorophenyl) -1- (1H-1,2,4-triazol-1-yl ) -2-butanol
- (2R,3R)-Voriconazole
- Voriconazole PI-6
- (2S,3R)-rel-2-(2,4-Difluorophenyl)-3-(5-fluoropyriMidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
- (2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
- (2R,3S/2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoro-4-pyriMidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (RaceMic Voriconazole)