ChemicalBook > CAS DataBase List > CARBONYL FLUORIDE
CARBONYL FLUORIDE
CARBONYL FLUORIDE
- CAS No.353-50-4
- Chemical Name:CARBONYL FLUORIDE
- CBNumber:CB9253542
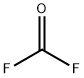
- Molecular Formula:CF2O
- Formula Weight:66.01
- MOL File:353-50-4.mol
CARBONYL FLUORIDE Property
- Melting point -114°C
- Boiling point -84°C
- Density 1,139 g/cm3
- solubility reacts with H2O
- form colorless gas
- color colorless
- Water Solubility instantly hydrolyzed by H2O [MER06]
- EWG's Food Scores 1
- FDA UNII 2NU89R5398
- EPA Substance Registry System Carbonic difluoride (353-50-4)
Safety
- Hazard Codes :T
- Risk Statements :8-23/24/25-34
- Safety Statements :3/7-9-36/37/39-38-45
- RIDADR :2417
- OEB :B
- OEL :TWA: 2 ppm (5 mg/m3), STEL: 5 ppm (15 mg/m3)
- Hazard Note :Highly Toxic
- HazardClass :2.3
- Hazardous Substances Data :353-50-4(Hazardous Substances Data)
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H280-H330
- Precautionary statements P410+P403-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501
CARBONYL FLUORIDE Chemical Properties,Usage,Production
- Description Carbonyl fluoride is a carboxy halide. It is colorless or light yellow, hygroscopic, compressed liquefiedgas, with a pungent, highly irritating and suffocating odor.Molecular weight=66.01; Specific gravity (H2O:1)=1.39at 2190℃; Boiling point=283℃; Freezing/Meltingpoint=2114℃; Relative vapor density (air=1)=2.29;Vapor pressure=55.4 atm. Hazard Identification (based onNFPA-704 M Rating System): Health 4, Flammability 0,Reactivity . Reacts with water.
- Chemical Properties Carbonyl fluoride is colorless or light yellow, hygroscopic, compressed liquefied gas. Pungent, highly irritating and suffocating odor.
- Physical properties Colorless gas; pungent odor; hygroscopic; unstable; liquid density 1.139 g/mL (at -114°C); liquefies at -83.1°C; solidifies at -114°C; decomposes in water.
- Uses Organic synthesis.
-
Preparation
Carbonyl fluoride is prepared by the reaction of carbon monoxide with fluorine gas or silver fluoride:
CO + F2 → COF2
Also, it may be produced by the action of carbon monoxide with bromine trifluoride, BrF3. - General Description A colorless gas with a pungent odor. Very toxic by inhalation. Prolonged exposure of the containers to fire or heat may result in violent rupturing and rocketing.
- Air & Water Reactions Reacts with water or steam to produces corrosive and toxic hydrofluoric acid fumes.
- Reactivity Profile CARBONYL FLUORIDE is an acid fluoride. Incompatible with water, with bases (including amines), with strong oxidizing agents, with alcohols. Reacts violently with hexafluoroisopropylideneaminolithium. High temperature causes decomposition to toxic carbon monoxide gas and fluorine. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
- Hazard Toxic by inhalation, strong irritant to skin. Lower respiratory tract irritant. Bone damage.
- Health Hazard Irritates lungs, causing delayed pulmonary edema. Slight gassing produces dryness or burning sensation in the throat, numbness, pain in the chest, bronchitis, and shortness of breath.
- Fire Hazard Special Hazards of Combustion Products: Toxic gas is generated when heated.
- Safety Profile A poison. Moderately toxic by inhalation. A powerful irritant. Hydrolyzes instantly to form HF on contact with moisture. See also CARBONYLS, HYDROFLUORIC ACID, and FLUORINE. Incompatible with hexafluoroisoprop ylideneamino-lithium. When heated to decomposition it emits toxic fumes of CO and F-. See CARBON MONOXIDE for fire and explosion hazard.
- Potential Exposure Carbonyl fluoride is a carboxy halide. The major source of exposure to COF2 results from the thermal decomposition of fluoro carbon plastics, such as PTFE in air. Carbonyl fluoride is used for synthesizing fluoroalkanes, difluoroisocyanates, and fluorinated alkyl isocyanates. It may have been used as a military poison gas.
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray. If frostbite has occurred, seek medical attention immediately; do NOT rub the affected areas or flushthem with water. In order to prevent further tissue damage,do NOT attempt to remove frozen clothing from frostbittenareas. If frostbite has NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
- storage Color Code—White stripe: Contact Hazard; Storeseparately; not compatible with materials in solid white category. Storage area must be absolutely dry.
- Shipping UN2417 Carbonyl fluoride, Hazard class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
- Incompatibilities Reacts with water to form toxic and corrosive HF gas. HF gas is highly reactive and forms explosive hydrogen gas on contact with metals. Do not use cast iron or malleable fittings with carbonyl fluoride. Carbonyl fluoride decomposes on heating above 450C producing toxic gases, including HF. Not compatible with hexafluoroisopropylidene-amino lithium; reaction may be dangerous.
- Toxics Screening Level The ITSL for carbonyl fluoride is 54 μg/m3 based on an 8-hour averaging time.
- Waste Disposal Return refillable compressed gas cylinders to supplier.
CARBONYL FLUORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
353-50-4, CARBONYL FLUORIDERelated Search:
- CARBONYL FLUORIDE 4-fluoro-5-oxo-2,4-bis(trifluoromethyl)-1,3-dioxolane-2-carbonyl fluoride Bicyclo[2.2.2]oct-2-ene-1-carbonyl fluoride (9CI) 1,3-Dioxolane-4-carbonyl fluoride, 2,2,4,5,5-pentafluoro- (9CI) 1,3-Cyclopentadiene-1-carbonyl fluoride, 2,3,4,5,5-pentafluoro- (9CI) Tricyclo[2.2.1.02,6]heptane-1-carbonyl fluoride, 7,7-dimethyl- (7CI,8CI) 1H-Pyrrole-3-carbonyl fluoride, 4-(difluoromethyl)-1-methyl- (9CI) Tricyclo[3.3.1.13,7]decane-1-carbonyl fluoride (9CI) Bicyclo[2.2.2]octane-1-carbonyl fluoride, 4-fluoro- (9CI) Bicyclo[2.2.1]heptane-2-carbonyl fluoride, 4,7,7-trimethyl-3-oxo-, (1R,4R)- (9CI) Bicyclo[2.2.1]heptane-7-carbonyl fluoride, 2,2-difluoro-1,7-dimethyl-, (1R,4R,7R)- (9CI) Bicyclo[2.2.1]heptane-7-carbonyl fluoride, 1,7-dimethyl-2-oxo-, (1R,4R,7R)- (9CI) 3-Cyclohexene-1-carbonyl fluoride, 2-methoxy-, (1S-cis)- (9CI) Bicyclo[2.2.1]heptane-1-carbonyl fluoride, 7,7-dimethyl-2-oxo-, (1S,4R)- (9CI) 1,3-Benzodioxole-4-carbonyl fluoride, 2,2-difluoro- (9CI) Bicyclo[2.2.2]octane-1-carbonyl fluoride (9CI) Bicyclo[2.2.1]heptane-1-carbonyl fluoride, 2,2-difluoro-7,7-dimethyl-, (1R,4R)- (9CI) Bicyclo[2.2.2]octane-1-carbonyl fluoride, 4-methyl- (9CI)
- FCOF
- CF2O
- 氟光气
- 氟氧化碳
- 碳酰氟
- 羰基氟
- 氟甲醯氟
- 氧氟化碳
- 氟化羰基
- 353-50-4
- Difluorophosgene
- COF2
- Fluorophosgene
- rcrawastenumberu033
- Rcra waste number U033
- fluoruredecarbonyle
- fluoroformylfluoride
- Fluoroformyl fluoride
- Fluophosgene
- Difluorooxomethane
- Difluoroformaldehyde
- carbonoxyfluoride(cof2)
- carbonoxyfluoride
- carbonicdifluoride
- CARBONYL DIFLUORIDE
- Carbon oxyfiuoride
- Carbonyl fluoride (COF2)
- Fluoroformic acid fluoride
- Difluoromethanone
- Difluoro ketone
- Carbonic acid difluoride
- Carbon oxyfluoride (R,T)
- Carbonylfluoride95%
- carbonfluorideoxide(cof2)
- carbonfluorideoxide
- carbondifluorideoxide
- Carbon oxyfluoride
- Carbon fluoride oxide (COF2)
- Carbon fluoride oxide
- Carbon difluoride oxide
- CARBONYL FLUORIDE