ChemicalBook > CAS DataBase List > APOMORPHINE HYDROCHLORIDE
APOMORPHINE HYDROCHLORIDE
APOMORPHINE HYDROCHLORIDE
- CAS No.314-19-2
- Chemical Name:APOMORPHINE HYDROCHLORIDE
- CBNumber:CB8357092
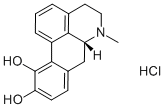
- Molecular Formula:C17H18ClNO2
- Formula Weight:303.78
- MOL File:314-19-2.mol
APOMORPHINE HYDROCHLORIDE Property
- Melting point >250℃
- storage temp. Store at RT
- solubility ≥1.08 mg/mL in EtOH with ultrasonic; ≥12.9 mg/mL in DMSO; ≥5.12 mg/mL in H2O
- form solid
- FDA UNII 9K13MD7A0D
- EPA Substance Registry System Apomorphine hydrochloride (314-19-2)
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H334-H312-H332-H317
- Precautionary statements P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312-P280-P302+P352-P312-P322-P363-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P261-P285-P304+P341-P342+P311-P501
APOMORPHINE HYDROCHLORIDE Chemical Properties,Usage,Production
- Originator Apomorphine hydrochloride,Nastech Pharmaceuticals Company, Inc.
- Uses Emetic.
- Uses (R)-(-)-Apomorphine Hydrochloride is a prototypical dopamine agonist. Potential treatment for Parkinson’s disease.
-
Manufacturing Process
2 Methods of producing of apomorphine
1. The apomorphine was obtained by dehydratation of morphine at heating to 120°C in the presence phosphoric acid and rendering of HCl gas over reaction mixture.
2. The morphine was converted to β-chloromorphine and then to dichlorodihydrodesoxymorphine at heating to 140°-150°C in the presence hydrochloric acid. Then apomorphine is obtained by dehydratation of dichlorodihydrodesoxymorphine. - brand name Apokyn (Vernalis).
- Therapeutic Function Emetic, Expectorant, Hypnotic, Antiparkinsonian, Dopamine agonist
- General Description Apomorphine hydrochloride,(6aR)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolone-10,11-diol hydrochloride (Apokyn), is awhite or off-white powder or crystal soluble in hot water(pKa=8.92). Apormorphine is an aporphine alkaloid of thebenzoquinoline class. Oral apomorphine is poorly absorbedand has a bioavailability of less than 4%. Upon subcutaneousadministration, apomorphine is completely absorbed. Within10 to 20 minutes, the maximum concentration of the drug isdistributed from the blood plasma to the CSF. Other potentialroutes of administration include continuous subcutaneous infusion,intravenous infusion, intranasal spray application,sublingual, and rectal administration.23 The agent is highlylipophilic in nature, allowing for rapid diffusion across theBBB after injection. Apomorphine has a short plasma halflife;however, clinical effects may last from 60 to 90 minutes.Apomorphine displays a significant degree of interpatientvariability in its pharmacokinetic profile. Studies of bothintravenous and subcutaneous injection routes found this variation was not attributable to body weight, age, gender,and duration of PD or L-DOPA dose/duration alone.Apomorphine is extensively metabolized. Hypothesizedroutes include sulfation, N-demethylation, glucuronidation,and oxidation. Subcutaneous injections of apomorphine arerenally and hepatically cleared, with the majority appearingto be renally cleared. Dosage adjustments are needed in bothliver and renal impairment. The activity of apomorphine isbelieved to be caused by stimulation of postsynaptic D1- andD2-type receptors within the caudate/putamen in the brain.Apomorphine is indicated for the acute, intermittent treatmentof hypomobility, “off” episodes (“end-of-dose wearingoff” and unpredictable on/off episodes) associated with advancedPD.
- Biological Activity Prototypical dopamine agonist (pK i values are 6.43, 7.08, 7.59, 8.36 and 7.83 for human recombinant D 1 , D 2L , D 3 , D 4 and D 5 receptors respectively). Produces biphasic effects on locomotor activity, and displays anti-Parkinsonian and neuroprotective actions following systemic administration in vivo .
- Clinical Use Treatment of refractory motor fluctuations in Parkinson’s disease
- Safety Profile Poison by intravenous andintraperitoneal routes. Mutation data reported. When heated to decomposition itemits very toxic fumes of NOx and HCl.
- Veterinary Drugs and Treatments Apomorphine is used primarily as an emetic in dogs, and is considered the emetic of choice for dogs by many clinicians. It is sometimes used in cats, but its use in this species is somewhat controversial.
-
Drug interactions
Potentially hazardous interactions with other drugs
Antihypertensives: enhanced hypotensive effect.
Domperidone: possible increased risk of ventricular arrhythmias.
5HT3 -receptor antagonists: possibly increased hypotensive effects with ondansetron.
Nitrates: enhanced hypotensive effect. - Metabolism After subcutaneous injection its fate can be described by a two-compartment model, with a distribution half-life of 5 (±1.1) minutes and an elimination half-life of 33 (±3.9) minutes. Clinical response correlates well with levels of apomorphine in the cerebrospinal fluid. Apomorphine is extensively metabolised in the liver, mainly by conjugation with glucuronic acid or sulfate; the major metabolite is apomorphine sulfate. It is also demethylated to produce norapomorphine. Most of a dose is excreted in urine, mainly as metabolites.
- storage Store at RT
APOMORPHINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(55)Suppliers
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
InvivoChem
- Tel:+1-708-310-1919<br/>+1-13798911105
- Email:sales@invivochem.cn
- Country:United States
- ProdList:6391
- Advantage:58
-
Supplier:
Henan Fengda Chemical Co., Ltd
- Tel:+86-371-86557731<br/>+86-13613820652
- Email:info@fdachem.com
- Country:China
- ProdList:20235
- Advantage:58
-
Supplier:
SHANGHAI KEAN TECHNOLOGY CO., LTD.
- Tel: +8613817748580
- Email:cooperation@kean-chem.com
- Country:China
- ProdList:40066
- Advantage:58
- Supplier: EMMX Biotechnology LLC
- Tel:888-539-0666
- Email:info@emmx.com
- Country:United States
- ProdList:8447
- Advantage:60
- Supplier: Beijing Solarbio Science & Tecnology Co., Ltd.
- Tel:010-50973186<br/>4009686088
- Email:3193328036@qq.com
- Country:China
- ProdList:18338
- Advantage:68
- Supplier: Shenzhen Polymeri Biochemical Technology Co., Ltd.
- Tel:+86-400-002-6226<br/>+86-13028896684;
- Email:sales@rrkchem.com
- Country:China
- ProdList:57422
- Advantage:58
- Supplier: Shenzhen Botel Biotechnology Co. Ltd.
- Tel:13316949107<br/>13316968096
- Email:1979313431@qq.com
- Country:China
- ProdList:9253
- Advantage:58
314-19-2, APOMORPHINE HYDROCHLORIDERelated Search:
- Apomorphine hydrochloride R-(-)-,APOMORPHINE HYDROCHLORIDE, HEMIHYDRATE,R-(-)-APOMORPHINE HYDROCHLORIDE HEMIHYDRATE Nicotinic acid APOMORPHINE HYDROCHLORIDE apomorphine S(+)-APOMORPHINE HYDROCHLORIDE HYDRATE,S(+)-APOMORPHINE HYDROCHLORIDE DOPAMINE RECEPTOR ANT APOMORPHINE HYDROCHLORIDE - REFERENCE SPECTRUM APOMORPHINE HYDROCHLORIDE HE APOMORPHINE HYDROCHLORIDE IMP. B (EP) AS HYDROCHLORIDE TRIHYDRATE:MORPHINE HYDROCHLORIDE TRIHYDRATE S(+)-APOMORPHINE HYDROCHLORIDE R(-)-10-METHOXY-11-HYDROXYAPORPHINE HYDROCHLORIDE rac-Apomorphine-13C-d3 HCl Apomorphine-13C-d3 HCl (S)-Apomorphine-13C-d3 HCl 6a-beta-Noraporphine-10,11-diol, 6-phenethyl-, hydrochloride R(-)-PROPYLNORAPOMORPHINE HCL (R)-6-ETHYL-5,6,6A,7-TETRAHYDRO-4H-DIBENZO[DE,G]QUINOLINE-10,11-DIOL HYDROCHLORIDE (-)-MDO-NPA HYDROCHLORIDE R(-)-CHLOROETHYLNORAPOMORPHINE HCL
- 医药原料
- C17H18NO2Cl
- 【禁售】(R)-(-)-APOMORPHINE HYDROCHLORIDE
- 盐酸阿扑吗啡
- 化合物 T19715
- ()-APOMORPHINE (HYDROCHLORIDE)
- 盐酸阿朴吗啡
- 烟酸阿朴吗啡
- 盐酸去水吗啡
- 阿扑吗啡
- 變嗎啡鹼鹽酸鹽
- 啡酸
- R-(-)-阿朴吗啡盐酸盐
- R(–)-10,11-二羟基阿朴啡
- R(-)-阿扑吗啡 盐酸盐
- 盐酸去水吗啡(阿扑吗啡盐酸盐、盐酸阿朴吗啡、R-(-)-阿朴吗啡盐酸盐、啡酸、R(–)-10,11-二羟基阿朴啡)
- 314-19-2
- KW-6500
- APL-130277
- APL130277
- APL 130277
- Apomorphine HydrochlorideQ: What is Apomorphine Hydrochloride Q: What is the CAS Number of Apomorphine Hydrochloride Q: What is the storage condition of Apomorphine Hydrochloride Q: What are the applications of Apomorphine Hydrochloride
- TAK251)
- Apomorphine HCl (APL130277
- (?)-Apomorphine
- Apomorphinhydrochlorid
- APOMORPHINE HYDROCHLORIDE USP/EP/BP
- APOMORPHINE HYDROCHLORIDE
- APOMORPHINE HCL
- UPRIMA
- R(-)-10,11-DIHYDROXYAPORPHINE HYDROCHLORIDE
- (R)-5,6,6A,7-TETRAHYDRO-6-METHYL-4H-DIBENZO[DE,G]QUINOLINE-10,11-DIOL HYDROCHLORIDE
- (R)-(-)-APOMORPHINE HYDROCHLORIDE
- g]quinoline-10,11-diol,5,6,6a,7-tetrahydro-6-methyl-4h-dibenzo[dhydrochlor
- g)quinoline-10,11-diol,5,6,6a,7-tetrahydro-6-methyl-4h-dibenzo(dhydrochlor
- apomorphiniumchloride
- apomorphinechloride
- 6a-beta-aporphine-10,11-diol,hydrochloride
- 11-diol,6-methyl-6a-beta-noraporphine-1hydrochloride
- 6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-10,11-diol hydrochloride
- R(–)-APO
- NSC 11442
- R()-Apomorphine hydrochloride,R(–)-10,11-Dihydroxyaporphine, R(–)-APO
- g)quinoline-10,11-diol,5,6,6a,7-tetrahydro-6-methyl-4h-dibenzo(dhydr
- Apomorphine hydrochoride
- 4H-Dibenzode,gquinoline-10,11-diol, 5,6,6a,7-tetrahydro-6-methyl-, hydrochloride, (6aR)-
- (-)-APO H CL
- (theta)-id
- (r)-id
- (-)-apomorphiniumhydrochloride