ChemicalBook > CAS DataBase List > Ko 143
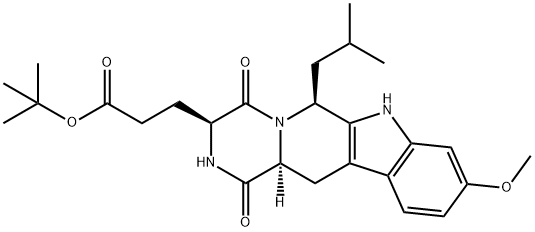
Ko 143
Ko 143
- CAS No.461054-93-3
- Chemical Name:Ko 143
- CBNumber:CB81562442
- Molecular Formula:C26H35N3O5
- Formula Weight:469.57
- MOL File:461054-93-3.mol
Ko 143 Property
- Melting point 147 ºC
- Boiling point 689.8±55.0 °C(Predicted)
- Density 1.24
- storage temp. Desiccate at -20°C
- solubility DMSO: >10mg/mL
- pka 13.31±0.60(Predicted)
- form powder
- color white to off-white
- Stability Stable for 2 years from date of purchase as supplied. Protect from exposure to moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months.
- UNSPSC Code 12352200
- NACRES NA.77
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P305+P351+P338
Ko 143 Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: K2144
- Product name : Ko143 hydrate
- Purity: ≥98% (HPLC)
- Packaging: 1MG
- Price: ₹12697.73
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: K2144
- Product name : Ko143 hydrate
- Purity: ≥98% (HPLC)
- Packaging: 5MG
- Price: ₹50379.55
- Updated: 2022/06/14
- Buy: Buy
Ko 143 Chemical Properties,Usage,Production
-
Description
Breast cancer resistance protein (BCRP) is an ATP-
binding cassette protein known also as ABCG2. While it normally functions as a high- capacity urate exporter in the renal system, it also acts as a xenobiotic transporter and contributes to multidrug resistance (e.g., to mitoxantrone) in certain types of cancer. BCRP is abundant at the intestinal epithelium and blood- brain barrier, potentially restricting the distribution of certain drugs. Ko 143 is a potent and selective inhibitor of BCRP, preventing the export of mitoxantrone and topotecan in breast cancer cell lines (EC50s = 23 and 26 nM, respectively). It is much less effective at the transporters P- glycoprotein and multidrug resistance- associated protein 1, MRP1. Ko 143 is effective in vivo in mice. - Uses A potent BCRP (breast cancer resistance protein) inhibitor
-
Uses
Ko143 hydrate has been used:
- to determine the role of ATP-binding cassette sub-family G member 2 (ABCG2), human embryonic kidney (HEK)-C1 and HEK-ABCG2 in tumor microenvironment
- to inhibit ABCG2 for sphere formation assay
- in calcein-AM efflux inhibition to monitor multidrug resistance protein (MRP)-function in kidney
- for cell viability assay
- Definition ChEBI: LSM-6260 is a member of beta-carbolines and a tert-butyl ester.
- Biological Activity Potent and selective breast cancer resistance protein multidrug transporter (BCRP) inhibitor (EC 90 = 26 nM). Displays > 200-fold selectivity over P-gp and MRP-1 transporters. Increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance.
- Biochem/physiol Actions Ko143 has been used as a positive control inhibitor on functions of breast cancer resistance protein (BCRP) using a BCRP prototypical substrate mitoxantrone. BCRP, an ABCG2 transporter, plays an important role in disposition of many drugs and environmental toxins. Ko143 displays > 200-fold selectivity over P-gp and MRP-1 transporters and thus is more specific than other known BCRP inhibitors such as fumitremorgin C and GF120918. It increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance. It blocks topotecan and ABZSO transport in a concentration-dependent manner.
- storage Store at -20°C
- References 1) Allen et al. (2002), Potent and specific inhibition of breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C; Mol. Cancer Ther., 1 417 2) Weidner et al. (2015), The Inhibitor Ko143 Is Not Specific for ABCG2; J. Pharmacol. Exp. Ther., 354 384 3) Palasuberniam et al. (2015), ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy; Sci. Rep., 5 13298 4) Moreno-Sanz et al. (2011), The ABC membrane transporter ABCG2 prevents access of FAAH inhibitor URB937 to the central nervous system; Pharmacol. Res., 64 359 5) Sabnis et al. (2017),?The Efflux Transporter ABCG2 Maintains Prostate Stem Cells;? Mol. Cancer Res., 15 128
Ko 143 Preparation Products And Raw materials
Raw materials
Preparation Products
Global(111)Suppliers
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Hefei Hirisun Pharmatech Co., Ltd
- Tel: +8615056975894
- Email:shawn@hirisunpharm.com
- Country:CHINA
- ProdList:9911
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
HangZhou RunYan Pharma Technology Co.,LTD.
- Tel: +8618882027439
- Email:sales2@runyanpharma.com
- Country:China
- ProdList:302
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
-
Supplier:
Guangzhou CATO Research Chemicals Inc.
- Tel:+86-020-81960175-617<br/>+8615602392859
- Email:Intermediate@cato-chem.com
- Country:China
- ProdList:6335
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39927
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
461054-93-3, Ko 143Related Search:
- p-Anisidine 4-Methoxybenzylchloride p-Anisaldehyde (Trifluoromethoxy)benzene 4-Methoxyphenylacetone 4-Methoxyphenylacetic acid Anisole 3-(METHOXYMETHOXY)BENZALDEHYDE Imatinib mesylate TERT-BUTYL PROPIONATE 3-Indolepropionic acid CYCLO(-GLY-GLU) CYCLO(-GLY-TRP) CYCLO(-ALA-GLU) 3-PIPERAZIN-2-YL-PROPIONIC ACID METHYL ESTER 3-PIPERAZIN-2-YL-PROPIONIC ACID Ko 143 Methoxy
- Intermediates & Fine Chemicals
- Inhibitors
- Heterocycles
- Chiral Reagents
- Pharmaceuticals
- 杂质对照品
- 抑制剂
- 合成有机化合物配体
- 细胞生物学试剂
- 细胞生物学
- 细胞信号和神经生物学
- C26H36N3O5
- C26H35N3O5
- 化合物KO 143,10 MM DMSO 溶液
- KO 143抑制剂
- 3-((3S,6S,12AS)-6-异丁基-9-甲氧基-1,4-二氧代-1,2,3,4,6,7,12,12A-八氢吡嗪并[1',2':1,6]吡啶并[3,4-B]吲哚-3-基)丙酸叔丁酯
- KO 143,BCRP抑制剂
- KO143HYDRATE>=98%(HPLC)
- KO 143 水合物
- 乳腺癌耐药蛋白抑制剂(BCRP抑制剂)
- 1,6]吡啶并[3,4-B]吲哚-3-丙酸叔丁酯]
- [(3S,6S,12AS)-1,2,3,4,6,7,12,12A-八氢-9-甲氧基-6-(2-甲基丙基)-1,4-二氧代吡嗪并[1',2'
- (3S,6S,12AS)-八氢-9-甲氧基-6-(2-甲基丙基)-1,4-二氧吡嗪并[1',2':1,6]吡啶并[3,4-B]吲哚-3-羧酸叔丁酯
- (3S,6S,12AS)-1,2,3,4,6,7,12,12A-八氢-9-甲氧基-6-(2-甲基丙基)-1,4-二氧代吡嗪并[1',2':1,6]吡啶并[3,4-B]吲哚-3-丙酸叔丁酯
- 461054-93-3
- Ko 143, 10 mM in DMSO
- Ko-143, BCRP inhibitor
- (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-( 2-methylpropyl)-1,4-dioxopyrazino[1',2':1,6]pyrido[3,4- b]indole-3-propanoic Acid tert-Butyl Ester
- (3s,6s,12as)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1',2':1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester
- 1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester
- (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1',2'
- tert-Butyl 3-((3S,6S,12aS)-6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino
- KO 143;KO-143;KO143
- tert-butyl 3-((3S,6S,12aS)-6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)propanoate
- (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-( 2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4- b]indole-3-propanoic acid 1,1-dimethylethyl ester hydrate
- tert-Butyl 3-((3S,6S,12aS)-6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7, 12,12a-octahydropyrazino[1
- Ko143 hydrate
- Ko143 ,99%
- Pyrazino[1'
- 6]pyrido[3
- 4-dioxo-
- 4-b]indole-3-propanoicacid
- 12aS)-
- 12a-octahydro-9-Methoxy-6-(2-Methylpropyl)-1
- Ko 143
- Pyrazino[1',2':1,6]pyrido[3,4-b]indole-3-propanoic acid, 1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxo-, 1,1-dimethylethyl ester, (3S,6S,12aS)-(Ko143)