ChemicalBook > CAS DataBase List > O-ALLYL-2,2,2-TRICHLOROACETIMIDATE
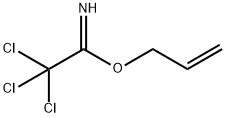
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE
- CAS No.51479-73-3
- Chemical Name:O-ALLYL-2,2,2-TRICHLOROACETIMIDATE
- CBNumber:CB8100737
- Molecular Formula:C5H6Cl3NO
- Formula Weight:202.47
- MOL File:51479-73-3.mol
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE Property
- Boiling point 54-70 °C/7.5-11.25 mmHg
- Density 1.333 g/mL at 25 °C
- refractive index n20/D 1.489
- Flash point 74 °C
- storage temp. 2-8°C
- solubility sol hexane, ether, dichloromethane.
- pka 2?+-.0.70(Predicted)
- form clear liquid
- color Colorless to Almost colorless
- BRN 1907084
- UNSPSC Code 12352101
Safety
- Hazard Codes :Xn
- Risk Statements :22-37/38-41
- Safety Statements :26-39
- WGK Germany :3
- HS Code :29252900
-
NFPA 704:
2 2 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H302-H315-H318-H335-H227
- Precautionary statements P210-P264-P302+P352+P332+P313+P362+P364-P305+P351+P338+P310-P403+P235-P501-P261-P280-P305+P351+P338
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE Price
More Price(1)
- Brand: TCI Chemicals (India)
- Product number: A2186
- Product name : Allyl 2,2,2-Trichloroacetimidate
- Purity: min. 98.0 %
- Packaging: 5G
- Price: ₹11100
- Updated: 2022/05/26
- Buy: Buy
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE Chemical Properties,Usage,Production
- Uses Reagent used for the protection of an alcohol as its allyl ether in the presence of base sensitive functionality.
- Application O-allyl-2,2,2-trichloroacetimidate can be used to release allyl groups under mild conditions; used to prepare nitrogen-containing intermediates.
- Preparation Sodium Hydride (0.5 g, 21 mmol) is slurried in anhydrous ether (20 mL) under a nitrogen blanket. Allyl alcohol (210 mmol) is introduced in ether (30 mL) dropwise with stirring to the slurry. After 20 min, the homogeneous solution is cooled to 0 °C and Trichloroacetonitrile (20 mL, 200 mmol) is added over 15 min. The mixture is allowed to warm to 20 °C over 60 min and then concentrated to a syrup. Pentane (20 mL) containing methanol (0.8 mL, 21 mmol) is added followed by vigorous shaking, filtration, and concentration of the filtrate and pentane washings (2 × 20 mL). The product imidate is obtained as a clear liquid (90-97%). No further purification is required. Alternatively, the imidate can be prepared by using sodium methoxide as a catalyst instead of sodium hydride. Other procedures for the preparation of allyl trichloroacetimidate have been reported.
O-ALLYL-2,2,2-TRICHLOROACETIMIDATE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(92)Suppliers
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19809
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
Aceschem Inc.
- Tel:+1-817863-6948<br/>+1-(817)863-6948
- Email:sales@aceschem.com
- Country:United States
- ProdList:19632
- Advantage:58
-
Supplier:
Changzhou AniKare Pharmatech Co., Ltd.
- Tel:+86-0519-8359-8696<br/>+8618018249389
- Email:sales@anikare.com
- Country:China
- ProdList:9875
- Advantage:58
51479-73-3, O-ALLYL-2,2,2-TRICHLOROACETIMIDATERelated Search:
- 高端化学
- 有机中间体
- 其他生化试剂
- 酰胺化合物
- 酰胺
- Amidates/Imidates
- Building Blocks
- Nitrogen Compounds
- Organic Building Blocks
- C5H6Cl3NO
- O-烯丙基-2,2,2-三氯乙酰亚胺酯
- 2,2,2-三氯亚氨逐乙酸烯丙酯
- 2,2,2-三氯乙酰胺烯丙酯
- 2,2,2-三氯乙酰胺烯丙酯 5G
- 三氯乙酰亚氨酸丙烯酯
- 51479-73-3
- <i>O-</i>Allyl 2,2,2-trichloroacetimidate
- Allyl Trichloroacetimidate
- ALLYL 2,2,2-TRICHLOROACETIMIDATE
- Ethanimidic acid, 2,2,2-trichloro-, 2-propen-1-yl ester
- Allyl 2,2,2-Trichloroacetimidate >
- O-ALLYL-2,2,2-TRICHLOROACETIMIDATE
- O-Allyl 2,2,2-trichloroacetimidate 96%
- 2,2,2-TRICHLOROACETIMIDIC ACID ALLYL ESTER
- 2,2,2-TRICHLORACETIMIDIC ACID ALLYLESTER