ChemicalBook > CAS DataBase List > lasiocarpine
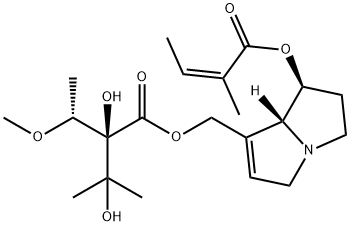
lasiocarpine
lasiocarpine
- CAS No.303-34-4
- Chemical Name:lasiocarpine
- CBNumber:CB7875229
- Molecular Formula:C21H33NO7
- Formula Weight:411.49
- MOL File:303-34-4.mol
lasiocarpine Property
- Melting point 96.5-97.0℃
- alpha D -4° (10% alc)
- Boiling point 531.2°C (rough estimate)
- Density 1.21±0.1 g/cm3 (20 ºC 760 Torr)
- refractive index 1.5614 (estimate)
- pka 11.99±0.29(Predicted)
- Water Solubility 6.754g/L(temperature not stated)
- Stability Stable, but is readily hydrolyzed in basic solution and readily oxidized by oxidizing agents. Reacts slowly with atmospheric oxygen.
- LogP 1.280
- EWG's Food Scores 1
- FDA UNII S770100Q96
- Proposition 65 List Lasiocarpine
- IARC 2B (Vol. 10, Sup 7) 1987
- EPA Substance Registry System Lasiocarpine (303-34-4)
Safety
- RIDADR :1544
- HazardClass :6.1(b)
- PackingGroup :III
- Hazardous Substances Data :303-34-4(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H301
- Precautionary statements P264-P270-P301+P310-P321-P330-P405-P501
lasiocarpine Chemical Properties,Usage,Production
- Description A hepatotoxic alkaloid found in Heliotropiurn lasiocarpurn, the base forms colourless leaflets from petroleum ether and has [α]20D - 4° (EtOH). It is readily soluble in C6H6 or EtOH but only slightly so in H20. On alkaline hydrolysis it furnishes angelic acid and heliotridine, C8H1302N, m.p. 116.5-118°C, forming a hydrochloride, m.p. 122-4°C and the benzoyl derivative hydrochloride, m.p. 180°C. On catalytic hydrogenation it yields a base, C13H230 2N, b.p. 123-5°Cj 8 mm; [α]D + 3.8° (EtOH), yielding a crystalline picrate, m.p. 157 _9°C, and lasiocarpic acid, C8H160 S, m.p. 95-7°C; [α]D + 10.6° (EtOH). The structure is therefore angelyllasiocarpylheliotridine, both hydroxyls in heliotridine being es terifie d
- Chemical Properties colourless to beige crystalline solid
- Uses Lasiocarpine is a hepatotoxic and carcinogenic food contaminant.
- General Description Colorless plates or beige crystalline solid.
- Air & Water Reactions Decomposes slowly on standing in air at room temperature. Insoluble in water.
- Reactivity Profile lasiocarpine is readily hydrolyzed by alkali. Reacts readily with oxidizing agents. Reacts slowly with atmospheric oxygen .
- Health Hazard ACUTE/CHRONIC HAZARDS: When heated to decomposition lasiocarpine emits toxic fumes of nitrogen oxides.
- Fire Hazard Flash point data for lasiocarpine are not available; however, lasiocarpine is probably combustible.
- References Men'shikov., Ber., 65,974 (1932)
lasiocarpine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(43)Suppliers
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
SHANGHAI KEAN TECHNOLOGY CO., LTD.
- Tel: +8613817748580
- Email:cooperation@kean-chem.com
- Country:China
- ProdList:40066
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
- Supplier: ALB Technology Limited
- Tel:702-983-3769
- Email:sales@albtechnology.com
- Country:United States
- ProdList:2993
- Advantage:55
- Supplier: Wuhan ChemFaces Biochemical Co., Ltd.
- Tel:18607101326<br/>15172504745
- Email:aileen@chemfaces.com
- Country:China
- ProdList:6905
- Advantage:55
- Supplier: Biyu Biotechnology (Shanghai) Co., ltd
- Tel:021-26018599
- Email:
- Country:China
- ProdList:270
- Advantage:56
- Supplier: EMMX Biotechnology LLC
- Tel:888-539-0666
- Email:info@emmx.com
- Country:United States
- ProdList:8447
- Advantage:60
- Supplier: Shanghai YuanYe Biotechnology Co., Ltd.
- Tel:021-61312847;<br/>18021002903
- Email:3008007409@qq.com
- Country:China
- ProdList:71829
- Advantage:60
303-34-4, lasiocarpineRelated Search:
- veratramine VERATRIDINE Xanthiazone menisdaurin daurisoline L-Hyoscyamine Daurinoline VERATRINE 2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one Xanthiside lasiocarpine ALLYL 2-ETHYLBUTYRATE ETHOXYPYRROLIDINE(S-3-) symphytine HELIOTRIDINE (S)-3-METHOXYPYRROLIDINE 2,3,5,7A-TETRAHYDRO-1H-PYRROLIZINE
- 分析试剂标准品
- C21H33NO7
- 化合物 T20524
- 毛果天芥菜鹼
- 毛果天芥菜碱
- 303-34-4
- Lasiocarpine-RM
- Lasiocarpine in methanol
- 2-Butenoic acid,2-methyl-,(1S,7aR)-7-[[(2R)-2,3-dihydroxy-2-[(1S)-1-methoxyethyl]-3-methyl-1-oxobutoxy]methyl]-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-ylester, (2Z)-
- Europine 7-angelate
- AI3-51770
- 7-Angelyleuropine
- (-)-Lasiocarpine
- 2-Butenoic acid, 2-methyl-, (1S,7aR)-7-[[(2R)-2,3-dihydroxy-2-[(1R)-1-methoxyethyl]-3-methyl-1-oxobutoxy]methyl]-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-yl ester, (2Z)-
- Lasiocarpin
- Nsc30625
- Aids-002693
- Aids002693
- 2-Methyl-2-butenoic acid 7-[[2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy]methyl]-2,3,5,7A-tetrahydro-1H-pyrrolizin-1-yl ester
- (Z)-2-Methyl-2-butenoic acid (1S)-7-[[[(2R)-2,3-dihydroxy-2-[(S)-1-methoxyethyl]-3-methylbutanoyl]oxy]methyl]-2,3,5,7aβ-tetrahydro-1H-pyrrolizin-1-yl ester
- 2-Butenoic acid, 2-methyl-, 7[[(2,3-dihydroxy-2-(1-methoxyethyl)-3-methyl-1-oxobutoxy] methyl]-2,3,5,7a-tetrahydro-1H-prrolizin-1-yl ester, [1S-[1alpha(Z), 7(2S*, 3R*), 7aalpha)]-
- lasiocarpine