ChemicalBook > CAS DataBase List > Diphenylamine
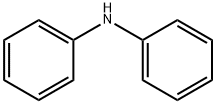
Diphenylamine
Diphenylamine
- CAS No.122-39-4
- Chemical Name:Diphenylamine
- CBNumber:CB7852949
- Molecular Formula:C12H11N
- Formula Weight:169.22
- MOL File:122-39-4.mol
Diphenylamine Property
- Melting point 52 °C
- Boiling point 302 °C(lit.)
- Density 1.16
- bulk density 610kg/m3
- vapor density 5.82 (vs air)
- vapor pressure 1 mm Hg ( 108 °C)
- refractive index 1.5785 (estimate)
- Flash point 307 °F
- storage temp. Store below +30°C.
- solubility alcohol: passes test
- form crystalline
- pka 0.79(at 25℃)
- color tan
- Odor Floral odor
- Water Solubility Slightly soluble. 0.03 g/100 mL
- Sensitive Air & Light Sensitive
- Merck 14,3317
- BRN 508755
- Exposure limits TLV-TWA 10 mg/m3 (ACGIH and MSHA).
- Dielectric constant 3.3(11℃)
- Stability Stable; may discolour on exposure to light. Incompatible with strong acids, strong oxidizing agents.
- LogP 3.82 at 20.2℃
- Indirect Additives used in Food Contact Substances DIPHENYLAMINE
- FDA 21 CFR 175.105; 175.300; 176.170; 176.180
- CAS DataBase Reference 122-39-4(CAS DataBase Reference)
- EWG's Food Scores 3-6
- FDA UNII 9N3CBB0BIQ
- NIST Chemistry Reference Diphenylamine(122-39-4)
- Pesticides Freedom of Information Act (FOIA) Diphenylamine
- EPA Substance Registry System Diphenylamine (122-39-4)
- UNSPSC Code 77101502
- NACRES NA.24
Safety
- Hazard Codes :T,N,F
- Risk Statements :23/24/25-33-50/53-52/53-39/23/24/25-11-51/53
- Safety Statements :28-36/37-45-60-61-28A-16-7
- RIDADR :UN 3077 9/PG 3
- OEB :B
- OEL :TWA: 10 mg/m3
- WGK Germany :3
- RTECS :JJ7800000
- F :8-10-23
- Autoignition Temperature :630 °C DIN 51794
- TSCA :Yes
- HS Code :2921 44 00
- HazardClass :6.1
- PackingGroup :III
- Hazardous Substances Data :122-39-4(Hazardous Substances Data)
- Toxicity :LD50 orally in Rabbit: 1120 mg/kg LD50 dermal Rabbit > 5000 mg/kg
-
NFPA 704:
1 2 0
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H301+H311+H331-H373-H410
- Precautionary statements P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P314
Diphenylamine Price
More Price(28)
- Brand: Sigma-Aldrich(India)
- Product number: 8.20528
- Product name : Diphenylamine
- Purity: for synthesis
- Packaging: 1IN28205280250
- Price: ₹1470.01
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.20528
- Product name : Diphenylamine
- Purity: for synthesis
- Packaging: 100G
- Price: ₹5330
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: CRM69121
- Product name : Diphenylamine
- Purity: certified reference material, TraceCERT?
- Packaging: 100MG
- Price: ₹12427.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.20528
- Product name : Diphenylamine
- Purity: for synthesis
- Packaging: 1KG
- Price: ₹12720
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.20528
- Product name : Diphenylamine
- Purity: for synthesis
- Packaging: 50KG
- Price: ₹118150
- Updated: 2022/06/14
- Buy: Buy
Diphenylamine Chemical Properties,Usage,Production
- Description Solutions of diphenylamine are used to treat apples a few days before harvest. Residue on apples’ surfaces of 10 ppm is permitted by regulation in Australia, Canada, and the United States.
- Chemical Properties white crystals or powder
- Chemical Properties Diphenylamine is a colorless monoclinic leafl et substance. It is used in the manufacture of a variety of substances, i.e., dyestuffs and their intermediates, pesticides, antihelmintic drugs, and as reagents in analytical chemistry laboratories.
- Uses Diphenylamine is used post-harvest to prevent superficial scald in apples in cold store.
-
Uses
Diphenylamine is used in the manufactureof dyes, as a stabilizer for nitrocelluloseexplosives, and as an analytical reagent forcolorimetric tests for nitrate and chlorate.
Other applications of this compoundinclude preventing postharvest deteriorationof apple and peer crops; as an antioxidant inrubber and elastomer industry and in the per fumery. As a stabilizer for propellants andexplosives, it binds their degradation prod ucts thus prolonging the storage time of suchpropellants. - Uses Diphenylamine is an aromatic amine that was shown to exhibit antioxidant activities and is now used as an anti-scald agent. It is also used in the manufacture of a variety of substances, for instance, dye stuffs and their intermediates, pesticides, anthelmintic drugs, and as reagents in analytical chemistry laboratories.
- Definition ChEBI: An aromatic amine containing two phenyl substituents. It has been used as a fungicide for the treatment of superficial scald in apples and pears, but is no longer approved for this purpose within the European Union.
- Definition diphenylamine: A colourless crystallinearomatic compound,(C6H5)2NH; m.p. 54°C. It is made byheating phenylamine (aniline) withphenylamine hydrochloride. It is asecondary amine and is both slightlyacidic (forming an N-potassium salt)and slightly basic (forming salts withmineral acids). Its derivatives are employedas stabilizers for syntheticrubber and rocket fuels.
- Synthesis Reference(s) The Journal of Organic Chemistry, 58, p. 6900, 1993 DOI: 10.1021/jo00076a063
- General Description Light tan to brown solid with a pleasant odor. Sinks in water.
- Air & Water Reactions Dust may be explosive if mixed with air in critical proportions and in the presence of a source of ignition [USCG, 1999]. Insoluble in water.
- Reactivity Profile Diphenylamine discolors in light. Diphenylamine can react violently with hexachloromelamine and trichloromelamine. Diphenylamine is incompatible with strong oxidizing agents and strong acids. Diphenylamine is also incompatible with iron and silver salts. Diphenylamine reacts with nitrogen oxides.
- Health Hazard Inhalation may irritate mucous membranes. Overexposure, including ingestion of solid or skin contact, may cause fast pulse, hypertension, and bladder trouble. Contact with dust irritates eyes.
-
Health Hazard
Diphenylamine is much less toxic than aniline. The acute oral toxicity is low. A doseof 3000 mg/kg was lethal to rats. At a concentration of >500 ppm, a diet fed to ratsfor over 7 months resulted in renal cysts inanimals. Its absorption through the skin andthe respiratory system is lower than that ofaniline. Exposure to its dusts caused changesin liver, spleen, and kidney in test animals.Industrial exposure to diphenylamine hascaused tachycardia, hypertension, eczema,and bladder symptoms in workers (Fairhall1957). Carcinogenicity of this compound isunknown. It showed an adverse reproduc tive effect in animals, causing developmentalabnormalities in urogenital system in pregnant rats.
LD50 value, oral guinea pig: 300 mg/kg. - Health Hazard Diphenylamine is highly toxic and is rapidly absorbed by the skin and through inhalation. It has caused anorexia, hypertension, eczema, and bladder symptoms. Experimental animals exposed to diphenylamine demonstrated cystic lesions but failed to demonstrate cancerous growth. Inhalation of diphenylamine dust may cause systemic poisoning. The symptoms of toxicity include, but are not limited to, anoxia, headache, fatigue, anorexia, cyanosis, vomiting, diarrhea, emaciation, hypothermia, bladder irritation, kidney, heart, and liver damage.
- Fire Hazard Noncombustible solid; autoignition temperature 634°C (1173°F); low reactivity.
- Flammability and Explosibility Non flammable
- Agricultural Uses Insecticide, Fungicide, Herbicide, Plant growth regulator: Topically in anti-screwworm mixtures, foliar application in a modified growth chamber to decrease ozone injury to leaves of apple, bean, muskmelon, petunia, and tobacco plants. To control weather fleck in tobacco and inhibit algae formation. To prolong the fresh appearance of snapdragons. Protect rice from the toxic effects of thiolcarbamate herbicides [83] . Not currently approved for use in EU countries (resubmitted) . Registered for use in the U.S. and other countries.
- Trade name NOSCALD DPA 31; NOSCALD DPA 283; SCALDIP; Z-876
- Safety Profile Poison by ingestion. Experimental teratogenic effects. Action similar to anhne but less severe. Combustible when exposed to heat or flame. Can react violently with hexachloromelamine or trichloromelamine. Can react with oxilzing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of NOx,. See also ANILINE, AMINES, and AROMATIC AMINES.
- Potential Exposure AgriculturalChemical; Mutagen; Reproductive Effector. DPA is used asa stabilizer for plastics including solid rocket propellants;antioxidant for polymers, greases, and industrial oils; in themanufacture of pharmaceuticals; pesticides, explosives, anddyes.
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended.
-
Environmental Fate
Diphenylamine is present in waste water from industrial
processes. Diphenylamine has been detected in milk of
animals (cow, sheep, goat, water buffalo) raised in Italy and
France.
Pseudokirchneriella subcapitata (Algae) growth was inhibited with a dose of 0.30 mg l-1. Aquatic invertebrates Daphnia magna showed an acute 48 h EC50 dose of 1.2 mg l-1. - Metabolic pathway The major metabolite of diphenylamine (DPA) identified in stored apples is a glucose conjugate of 4-hydroxydiphenylamine, and additional metabolites, characterized as glycosyl conjugates of 2-hydroxy- DPA, 3-hydroxy-DPA, 4-hydroxy-DPA, or dihydroxy- DPA, are also detected along with their intact (i.e. non-conjugated) forms in apple pulp.
- storage Diphenylamine should be protected from physical damage. Storage of diphenylamine outside or a detached area is preferred. Inside storage should be in a standard flammable liquids storage room or cabinet. Diphenylamine should be kept separately from oxidizing materials and incompatible chemical substances. Storage and work areas should be no smoking areas. Diphenylamine should be kept protected from light.
- Shipping Toxic solids, flammable, organic, n.o.s. requiresa shipping label of “POISONOUS/TOXIC MATERIALS.”It falls into Hazard Class 6.1.
- Purification Methods Crystallise diphenylamine from pet ether, MeOH, or EtOH/water. Dry it under vacuum. [Beilstein 12 H 174, 12 IV 271.]
- Degradation Diphenylamine is an anti-oxidant and therefore reacts with oxygen under conditions of use. It darkens on exposure to sunlight. Aqueous photolysis is pH and oxygen dependant (Lopez et al., 1980). It is converted into carbazole (2) and hydrogen peroxide in the presence of dissolved oxygen. In degassed solution it is converted into carbazole (2) and tetrahydrocarbazole (3) (see Scheme 1).
- Incompatibilities Dust may form an explosive mixturewith air. Incompatible with strong acids, strong oxidizers,aldehydes, organic anhydrides, isocyanates, hexachloromelamine, trichloromelamine. Reacts with nitrogen oxidesforming n-nitrosodiphenylamine and heat-, friction-, andshock-sensitive nitro products
- Precautions Students and occupational workers should be careful during use and handling of diphenylamine. Workers should wear impervious protective clothing, including boots, gloves, a laboratory coat, apron or coveralls, as appropriate, to prevent skin contact. Finely dispersed particles of diphenylamine form explosive mixtures in air. Diphenylamine is very harmful on exposures by swallowing, inhalation, and/or skin absorption. Diphenylamine causes irritation to the skin, eyes, and respiratory tract, and causes blood vascular changes leading to methemoglobinemia.
Diphenylamine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(404)Suppliers
-
Supplier:
Shanghai UCHEM Inc.
- Tel:+862156762820<br/>+86-13564624040
- Email:sales@myuchem.com
- Country:China
- ProdList:7906
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12810
- Advantage:58
-
Supplier:
Shaanxi Haibo Biotechnology Co., Ltd
- Tel: +undefined18602966907
- Email:qinhe02@xaltbio.com
- Country:China
- ProdList:997
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418679<br/>+8618949832763
- Email:info@tnjchem.com
- Country:China
- ProdList:2986
- Advantage:55
-
Supplier:
Tianjin Zhongxin Chemtech Co., Ltd.
- Tel:86-22-66880623<br/>+8618622897568
- Email:sales@tjzxchem.com
- Country:China
- ProdList:572
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
QINGDAO HONG JIN CHEMCIAL CO.,LTD.
- Tel:532-83657313<br/>18663969289;
- Email:hjt@hong-jin.com
- Country:China
- ProdList:227
- Advantage:58
Related articles
122-39-4, DiphenylamineRelated Search:
- 3-Methoxydiphenylamine 4-Aminodiphenylamine Benzidine Aniline Dicyclohexylcarbodiimide 4-CHLORODIPHENYLAMINE N-(2,4-Dichlorophenyl)benzenamine Levocetetrizine N-Oxide (RS)-2-[2-[4-[(2-Chloro-phenyl)phenylMethyl]piperazin-1-yl]ethoxy]aceticAcidDihydrochloride Cetirizine Ethyl Ester (USP RC A) Cetirizine Glycerol Ester Cetirizine EP Impurity E 2(2-HYDROXYETHOXY)ACETAMIDE rac Cetirizine N-Oxide > 70% by HPLC (Mixture of Diastereomers) 1,4-BIS[(4-CHLOROPHENYL)PHENYLMETHYL]PIPERAZINE DIHYDROCHLORIDE Cetirizine EP Impurity B(base) (2-CHLORO-PHENYL)-PHENYL-AMINE 4-Chlorobenzophenone
- K00001
- bc0001
- 122-39-4
- TLC Visualization Reagents (by application)
- TLC Visualization Reagents (alphabetic sort)
- Indicators
- TLC Reagents, D-F
- Redox IndicatorsDerivatization Reagents TLC
- Nitrogen-containing compoundsTitration
- Derivatization Reagents TLC
- Chemical Class
- Aromatics
- Amines
- Intermediates of Dyes and Pigments
- Organics
- Pesticides
- Fungicides
- D
- Allergens
- Proposed Banning
- Other AdditivesEuropean Community: ISO and DIN
- DIO - DIZCosmetics
- Bridged diphenylsAlphabetic
- 76/768/EEC Annex VIII
- Nitrogen Compounds
- Routine Reagents
- Essential Chemicals
- C11 to C38
- ACS GradeNitrogen Compounds
- DAlphabetic
- Alpha sort
- Bridged diphenylsPesticides&Metabolites
- Thiophenes
- Solutions and Reagents
- Research Essentials
- Inorganic Salts
- Essential Chemicals
- DIG-DY
- Cell Biology
- Bioactive Small Molecules
- ACS Grade
- 高端化学
- 有机类
- 化工
- 无机产品
- 其他中间体材料
- 有机产品
- 杂质对照品
- 生化试剂
- 食品和饮料标准品
- 通用试剂
- 其他产品
- 有机原料
- 有机中间体
- 化工原料
- 二苯胺优势代理
- 农药中间体
- 胺类