ChemicalBook > CAS DataBase List > Bekanamycin
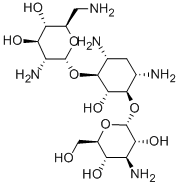
Bekanamycin
Bekanamycin
- CAS No.4696-76-8
- Chemical Name:Bekanamycin
- CBNumber:CB7432846
- Molecular Formula:C18H37N5O10
- Formula Weight:483.51
- MOL File:4696-76-8.mol
Bekanamycin Property
- Melting point 178-182° (dec)
- alpha D18 +130° (c = 0.5 in water); D21 +114° (c = 0.98 in water)
- Boiling point 580.49°C (rough estimate)
- Density 1.3771 (rough estimate)
- refractive index 1.7600 (estimate)
- storage temp. under inert gas (nitrogen or Argon) at 2–8 °C
- solubility Aqueous Acid (Slightly), Methanol (Slightly, Heated, Sonicated)
- pka 13.07±0.70(Predicted)
- form Solid
- color Crystals
- Stability Hygroscopic
- FDA UNII 15JT14C3GI
- ATC code J01GB13
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315;H319;H335
- Precautionary statements P261;P264;P271;P280;P302+P352;P304+P340;P305+P351+P338;P312;P321;P332+P313;P337+P313;P362;P403+P233;P405;P501
Bekanamycin Chemical Properties,Usage,Production
- Description Bekanamycin, kanamycin B, was found in the culture broth of Streptomyces kanamyceticus by Umezawa et al. in 1957. It shows the same antibacterial spectrum as kanamycin but with stronger activity. The total synthesis of bekanamycin was completed by Umezawa et al. in 1968 and the knowledge gained from its synthesis was successfully applied to the synthesis of dibekacin.
- Originator Kanendomycin,Meiji Seika,Japan,1969
- Uses Kanamycin B (cas# 4696-76-8) is a compound useful in organic synthesis.
- Definition ChEBI: Kanamycin B is a member of kanamycins. It is a conjugate base of a kanamycin B(5+).
-
Manufacturing Process
200 liters of the medium containing 2.0% starch, 1.0% soybean meal, 0.05%
KCl, 0.05% MgSO4·7H2O, 0.3% NaCl, 0.2% NaNO3 was placed in the 400 liter
fermenter, the pH was adjusted to 7.5, and the medium was then sterilized
(pH after the sterilization was 7.0) for 30 minutes at 120°C, inoculated with
1,000 ml of 40 hour shake-cultured broth of S. kanamyceticus (a selected
subculture of K2-J strain) and tank-cultured at 27°-29°C. As antifoam,soybean oil (0.04%)and silicone (0.04%) were added. The broth after 48
hours was found to contain 250 mcg/ml of kanamycin.
A portion (950 ml) of the rich eluate was adjusted to pH 6.0 by the addition of sulfuric acid. Ultrawet K (7.0 g) in 70 ml water was added slowly to the neutralized eluate to precipitate kanamycin B dodecylbenzenesulfonate which was collected by filtration after adding filter aid (Dicalite). The cake was washed with water and extracted with 100 ml methanol. After filtering and washing with methanol, sulfuric acid was added to the filtrate until no more kanamycin B sulfate precipitated. After addition of an equal volume of acetone to provide more complete precipitation, the kanamycin B sulfate was collected by filtration, washed with methanol and dried in vacuo at 50°C. - Therapeutic Function D-Streptamine, O-3-amino-3-deoxy-α-D-glucopyranosyl-(1- 6)-O-[2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl-(1-4)]-2-deoxy sulfate (1:1)
-
Antimicrobial activity
A component of the mixture of kanamycins produced by
Streptomyces kanamyceticus. It is approximately twice as active
as kanamycin A and is twice as toxic. It is not active against
amikacin-resistant strains of MRSA. It is poorly active against
Ps. aeruginosa.
The pharmacokinetics and uses are similar to those of kanamycin. A 0.5% ophthalmic solution has been used to treat gonococcal ophthalmia neonatorum. It is available in Japan. - Safety Profile Poison by intravenous route.Moderately toxic by intraperitoneal and subcutaneousroutes. When heated to decomposition it emits toxicfumes of NOx.
- Purification Methods A small quantity of kanamycin B (24mg) can be purified on a small Dowex-1 x 2 column (6 x 50mm); the required fraction is evaporated to dryness and the residue crystallised from EtOH containing a small amount of H2O. [Umezawa et al. Bull Chem Soc Jpn 42 537 1969.] It has been crystallised from H2O by dissolving ~1g in H2O (3mL), adding Me2NCHO (3mL) and setting aside at 4o overnight. The needles are collected and dried to constant weight at 130o. It has also been recrystallised from aqueous EtOH. It is slightly soluble in CHCl3 and isoPrOH. [IR: Wakazawa et al. J Antibiot 14A 180, 187 1961, Ito et al. J Antibiot 17 A 189 1964, Beilstein 18 III/IV 7631.]
Bekanamycin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(156)Suppliers
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81139210<br/>+86-18192627656
- Email:1059@dideu.com
- Country:China
- ProdList:3003
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
Finetech Industry Limited
- Tel:+86-27-87465837<br/>+8618971612321
- Email:info@finetechnology-ind.com
- Country:China
- ProdList:9642
- Advantage:58
-
Supplier:
Baoji Guokang Healthchem co.,ltd
- Tel:+8615604608665<br/>15604608665
- Email:dominicguo@gk-bio.com
- Country:CHINA
- ProdList:9414
- Advantage:58
4696-76-8, BekanamycinRelated Search:
- Glycine ALTRENOGEST KANAMYCIN AMIKACIN Tris(hydroxymethyl)aminomethane 6-Aminocaproic acid Amikacin Disulfate 3-Aminophenol Kanamycin sulfate BEKANAMYCIN SULFATE,BEKANAMYCIN SULFATE SALT 3'-Deoxyneamine 4-O-[6-Amino-2-[(aminocarbonyl)amino]-2,3,6-trideoxy-α-D-ribo-hexopyranosyl]-6-O-(3-amino-3-deoxy-α-D-glucopyranosyl)-2-deoxy-D-streptamine 2,6-DiaMino-2,3,6-trideoxy-D-ribo-Hexose Dihydrochloride Tobramycin (1'R,3'S,3S,5R,6R)-5-AMINO-2-AMINOMETHYL-6-(4,6-DIAMINO-2,3-DIHYDROXY-CYCLOHEXYLOXY)-TETRAHYDRO-PYRAN-3,4-DIOL Ethambutol Tobramycin Impurity KASUGAMYCIN
- API
- Pharmaceutical Intermediates
- Inhibitors
- 对照品-杂质对照品
- 抑制剂
- 生化试剂
- 原料药
- 其他原料
- 小分子抑制剂,天然产物
- C18H37N5O10
- 新卡霉素
- 妥布霉素杂质1 (妥布霉素杂质A )(卡那霉素B)
- 卡那霉素B对照品
- 卡那霉素杂质3
- 妥布霉素 EPA
- (2R,3S,4R,5R,6R)-5-氨基-2-(氨基甲基)-6-(((1R,2S,3S,4R,6S)-4,6-二氨基-3-(((2S ,3R,4S,5S,6R)-4-氨基-3,5-二羟基-6-(羟甲基)四氢-2H-吡喃-2-基)氧基)-2-羟基环己基)氧基)四氢-2H-吡喃- 3,4-二醇
- 卡那霉素杂质B
- 妥布霉素EP杂质A
- 卡那霉素 B?, >98%
- 卡那霉素B
- 4696-76-8
- New kanamycin
- Kanamycin B, EvoPure?
- 4-O-(3-amino-3-deoxy-α-D-glucopyranosyl)-2-deoxy-6-O-(2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl)-L-streptamine (kanamycin B)
- Kanamycin Impurity 3
- Kanamycine B
- Tobramycin EP Impurity A
- Kanamycin B base
- Kanamycin Impurity B
- Bekanamycin USP/EP/BP
- (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-(((1R,2S,3S,4R,6S)-4,6-diamino-3-(((2S,3R,4S,5S,6R)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)tetrahydro-2H-pyran-3,4-diol
- Tobramycin Impurity 1 (Tobramycin EP Impurity A) (Kanamycin B)
- D-Streptamine, O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→6)-O-[2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl-(1→4)]-2-deoxy-
- Bekanamycin, >98%
- kanamycin/kanamycin sulfate
- O-3-AMINO-3-DEOXY-D-GLUCOPYRANOSYL-(1,AT THE RATE4)-O-(2,6-DIAMINO-2,3,6TRIDEOXY-D-PER
- aminodeoxykanamycin
- 2’-amino-2’-deoxykanamycin
- Kanamycin b (intermediates)
- KANAMYCIN B
- NK-1006
- NK 1006
- Bekanamycin
- (2R,3S,4R,5R,6R)-5-Amino-2-(aminomethyl)-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2S,3R,4S,5S,6R)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxyoxane-3,4-diol
- nebramycinv
- nebramycinfactor5
- kdm
- kanendomycin
- d-streptamine,o-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-4)-o-(2,6-diamino-2,