ChemicalBook > CAS DataBase List > (4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE
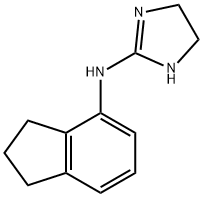
(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE
(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE
- CAS No.40507-78-6
- Chemical Name:(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE
- CBNumber:CB7389957
- Molecular Formula:C12H15N3
- Formula Weight:201.27
- MOL File:40507-78-6.mol
(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE Property
- Melting point 109-113°
- Boiling point 329.2±52.0 °C(Predicted)
- Density 1.30±0.1 g/cm3(Predicted)
- storage temp. Store at -20°C
- solubility Chloroform (Slightly), DMSO (Slightly)
- form Solid
- pka 9.01±0.10(Predicted)
- color White to Off-White
- FDA UNII L0U38EHD86
- ATC code R01AA15
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE Chemical Properties,Usage,Production
- Originator Farial,Nordmark-Werke,W. Germany,1980
- Uses Indanazoline is a sympathomimetic imidazoline derivative. Cyclopentamine showed vasoconstrictive action after local or intravenous applications.
- Definition ChEBI: Indanazoline is a member of indanes.
-
Manufacturing Process
38.5 g (0.1 mol) of N-4-indanyl thiourea are dissolved in 250 cc of methanol.
42,6 g (0.3 mol) of methyl iodide are added thereto and the mixture is
refluxed for 2,5 hours. The mixture thereafter is cooled and the solvent is
removed in a rotation evaporator in a vacuum. Thus, 57.5 g of N-4-indanyl-S_x0002_methylisothiuronium hydroiodide (86% of theoretical) are obtained. Melting
point 144°C to 146°C.
33.49 (0.1 mol) of N-4-indanyl-S-methylisothiuronium hydroiodide are mixed with 9.0 g (0.15 mol) of anhydrous ethylenediamine. The mixture is slowly heated to 80°C and heating is continued until the termination of the formation of methylmercaptan (about 4 hours). After cooling the residue is dissolved in 2N hydrochloric acid and the solution is extracted with chloroform. The extract is discarded and the aqueous phase is rendered alkaline by the addition of 10% soda lye. The resulting solution is extracted with chloroform and the extract is washed with water, dried over anhydrous sodium sulfate and the solvent is removed. An oily residue is obtained which upon standing soon crystallizes.
The product is recrystallized from petroleum ether having a boiling range of 100°C to 140°C in the presence of activated carbon. Thus, 11.1 g of 2-(4- indanylamino)-2-imidazoline (55% of theoretical) are obtained as the free base. Melting point 109°C to 113°C. - Therapeutic Function Nasal decongestant
(4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(36)Suppliers
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32202
- Advantage:58
-
Supplier:
Hangzhou MolCore BioPharmatech Co.,Ltd.
- Tel:+86-057181025280;<br/>+8617767106207
- Email:sales@molcore.com
- Country:China
- ProdList:49734
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel: +8613564774135
- Email:zijue.cai@tsbiochem.com
- Country:United States
- ProdList:19889
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: J & K SCIENTIFIC LTD.
- Tel: 18210857532
- Email:jkinfo@jkchemical.com
- Country:China
- ProdList:96815
- Advantage:76
- Supplier: Chemsky (shanghai) International Co.,Ltd
- Tel:021-50135380
- Email:shchemsky@sina.com
- Country:China
- ProdList:15402
- Advantage:60
- Supplier: Twochem Co.Ltd.
- Tel:021-58111628<br/>15800915896
- Email:sales@twochem.com
- Country:China
- ProdList:1788
- Advantage:58
- Supplier: Shenzhen Polymeri Biochemical Technology Co., Ltd.
- Tel:+86-400-002-6226<br/>+86-13028896684;
- Email:sales@rrkchem.com
- Country:China
- ProdList:57422
- Advantage:58
- Supplier: Tianjin Kaifu Pharmaceutical Technology Co., Ltd.
- Tel:18081075745
- Email:chemflowtech@sina.com
- Country:China
- ProdList:1886
- Advantage:58
- Supplier: Chunchuang (Wuhan) Technology Co., Ltd
- Tel: 15727060112
- Email:yutianchun2007@126.com
- Country:China
- ProdList:10000
- Advantage:58
40507-78-6, (4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINERelated Search:
- 抑制剂
- 化合物 T19371
- 印咪唑啉
- 茚唑啉
- 40507-78-6
- 4-Indanyl-AI
- 1H-Imidazol-2-amine, N-(2,3-dihydro-1H-inden-4-yl)-4,5-dihydro-
- 2-(4-IndanaMino)-2-iMidazoline
- N-(2,3-Dihydro-1H-inden-4-yl)-4,5-dihydro-1H-imidazol-2-amine
- indanazolin
- (4,5-DIHYDRO-1H-IMIDAZOL-2-YL)-4-INDANYLAMINE
- INDANAZOLINE