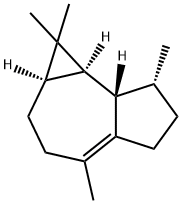
ChemicalBook > CAS DataBase List > (+)-Ledene
(+)-Ledene
(+)-Ledene
- CAS No.21747-46-6
- Chemical Name:(+)-Ledene
- CBNumber:CB7153554
- Molecular Formula:C15H24
- Formula Weight:204.35
- MOL File:21747-46-6.mol
(+)-Ledene Property
- Melting point 269°C
- Boiling point 268-270 °C (lit.)
- Density 0.927 g/mL at 20 °C (lit.)
- refractive index n
20/D 1.504 - storage temp. Refrigerator
- solubility Chloroform (Sparingly), Ethanol (Slightly)
- form Oil
- color Colourless
- optical activity [α]20/D +68±2°, c = 10% in ethanol
- BRN 2554786
- LogP 6.447 (est)
- FDA UNII 236ZZ41F70
- UNSPSC Code 12352002
- NACRES NA.22
Safety
- Safety Statements :23-24/25
- WGK Germany :3
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
(+)-Ledene Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 61770
- Product name : (+)-Ledene
- Purity: ≥95.0% (sum of enantiomers, GC)
- Packaging: 250MG
- Price: ₹11847.08
- Updated: 2022/06/14
- Buy: Buy
(+)-Ledene Chemical Properties,Usage,Production
- Description (+)-Ledene, also called leden or viridiflorene, is an organic compound that belongs to the group of 5, 10-cycloaromadendrane sesquiterpenoids. These compounds are derived from the aromadendrane skeleton through a cyclization reaction between carbon atoms 5 and 10. (+)-Ledene is mainly found in saliva and is considered to have low solubility in water and a relatively neutral nature. In cellular environments, (+)-ledene is predominantly present in the cell membrane (predicted based on logP) and cytoplasm.
- Uses (+)-Ledene is an essential oil that is synthesized by viridiflorene synthase. It has been studied as an antioxidant, antimicrobial, antibiofilm and to treat various cancers.
- Definition ChEBI: Viridiflorene is a carbotricyclic sesquiterpene obtained from several natural sources, including Australian Tea Tree oil (Melaleuca alternifolia.) It is a sesquiterpene and a carbotricyclic compound.
- General Description (+)-Ledene is a naturally occurring sesquiterpene that can be prepared from (+)-aromadendrene via isomerization.
-
References
[1] DUC N. TRAN Prof.??Dr. N C. Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (?)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene[J]. Chemistry - A European Journal, 2014. DOI:10.1002/chem.201403082.
[2] S. L. GWALTNEY K. J S S T Sakata. Bridged to Fused Ring Interchange. Methodology for the Construction of Fused Cycloheptanes and Cyclooctanes. Total Syntheses of Ledol, Ledene, and Compressanolide[J]. The Journal of Organic Chemistry, 1996. DOI:10.1021/jo961005a.
(+)-Ledene Preparation Products And Raw materials
Raw materials
Preparation Products
Global(49)Suppliers
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8772
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:52924
- Advantage:58
-
Supplier:
SHANGHAI KEAN TECHNOLOGY CO., LTD.
- Tel: +8613817748580
- Email:cooperation@kean-chem.com
- Country:China
- ProdList:40066
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
- Supplier: Meryer (Shanghai) Chemical Technology Co., Ltd.
- Tel:021-61259108<br/>18621169109
- Email:market03@meryer.com
- Country:China
- ProdList:40228
- Advantage:62
21747-46-6, (+)-LedeneRelated Search:
- CYCLOOCTENE (+)-Ledene CIS-1,2-DIMETHYLCYCLOPROPANE ETHYLIDENECYCLOPENTANE CYCLOHEPTENE 3-METHYLCYCLOOCTENE 1-METHYL-1-CYCLOHEPTENE 1-METHYL-1-CYCLOOCTENE METHYLENECYCLOPENTANE (1,2-DIMETHYLPROPYL)CYCLOPROPANE TRANS-1,2-DIMETHYLCYCLOPROPANE 2,3-DIMETHYL-2-OCTENE CIS-1-ETHYL-2-METHYLCYCLOPROPANE CIS-1,2-DIETHYLCYCLOPROPANE 2,3-DIMETHYL-2-HEPTENE (1-METHYLBUTYL)CYCLOPROPANE SEC-BUTYLCYCLOPROPANE
- 杂质对照品
- 抑制剂
- 高端化学
- 标准品
- 植物提取物
- Chiral Building Blocks
- Asymmetric Synthesis
- Complex Molecules
- 愈创奥杂质4
- 化合物 T25652
- (1S,2R,4R,11R)-3,3,7,11-四甲基三环[6.3.0.02.4]十一碳-7-烯
- (+)-喇叭烯
- 21747-46-6
- Guaiazulene Impurity 4
- (+)-Ledene
- 95.0% (sum of enantiomers, GC)
- 1H-Cycloprop[e]azulene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-, (1aR,7R,7aS,7bR)-
- (1aS,7S,7aS,7bS)-1,1,4,7-tetramethyl-1a,2,3,5,6,7,7a,7b-octahydrocyclopropa[e]azulene
- Varidiflorene
- 1H-Cycloprop[e]azulene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1aalpha,7alpha,7abeta,7balpha)]-
- 1,1,4,7-Tetramethyl-1a,2,3,5,6,7,7a,7b-octahydro-1H-cyclopropa[e]azulene
- LEDENE, (+)-(RG)
- Einecs 244-565-3
- (1Ar-(1aalpha,7alpha,7abeta,7balpha))-1A,2,3,5,6,7,7A,7B-octahydro-1,1,4,7-tetramethyl-1H-cycloprop(E)azulene
- (1aR)-1aβ,2,3,5,6,7,7aα,7bβ-Octahydro-1,1,4,7β-tetramethyl-1H-cyclopropa[e]azulene
- (1aR)-1a,2,3,5,6,7,7aα,7bβ-Octahydro-1,1,4,7β-tetramethyl-1H-cycloprop[e]azulene
- (+)-LEDENE 95+% MIXTURE OF ISOMERS
- (1S,2R,3R,11R)-3,3,7,11-TETRAMETHYL-TRICYCLO[6.3.0.0(2.4)]UNDEC-7-ENE
- [1aR-(1aalpha,7alpha,7abeta,7balpha)]-1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-1H-cycloprop[e]azulene
- (+)-Ledene >=95.0% (sum of enantiomers, GC)
- Viridflorene