ChemicalBook > CAS DataBase List > 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
- CAS No.290297-26-6
- Chemical Name:2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
- CBNumber:CB71483126
- Molecular Formula:C30H32F6N4O
- Formula Weight:578.59
- MOL File:290297-26-6.mol
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Property
- Melting point 156.2-160.0 °C
- Boiling point 597.4±50.0 °C(Predicted)
- Density 1.255
- storage temp. 2-8°C
- solubility Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly)
- pka 7.89±0.38(Predicted)
- form Solid
- color White to Off-White
- InChIKey WAXQNWCZJDTGBU-UHFFFAOYSA-N
- SMILES C(N(C)C1=C(C2C=CC=CC=2C)C=C(N2CCN(C)CC2)N=C1)(=O)C(C1C=C(C(F)(F)F)C=C(C(F)(F)F)C=1)(C)C
- FDA UNII 7732P08TIR
- NCI Drug Dictionary netupitant
- UNSPSC Code 12352200
- NACRES NA.77
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319
- Precautionary statements P302+P352-P305+P351+P338
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: SML2755
- Product name : Netupitant
- Purity: ≥98% (HPLC)
- Packaging: 10MG
- Price: ₹9290.7
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SML2755
- Product name : Netupitant
- Purity: ≥98% (HPLC)
- Packaging: 50MG
- Price: ₹37484.7
- Updated: 2022/06/14
- Buy: Buy
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Chemical Properties,Usage,Production
- Description Netupitant, originally developed by Helsinn Healthcare and later licensed to Eisai, Inc., was approved in the USA in October 2014 for the treatment of chemotherapy-induced nausea and emesis. Akynzeo ® is a fixed-dose combination of the new drug netupitant and the previously-approved 5-HT3 antagonist palonosetron. While palonosetron obtained approval previously for treating nausea and emesis occurring within the first 24 hours (acute phase) after chemotherapy, netupitant provides a synergistic effect with palonosetron, assisting in prevention of nausea and emesis in later stages following chemotherapy (25–120 h after chemotherapy treatment). Several clinical trials showed that this combination of netupitant and palonosetron (Akynzeo ?), in comparison to treatment with palonosetron treatment alone, led to an improved percentage of patients in all phases who did not experience any nausea and emesis after undergoing chemotherapy. Netupitant itself joins the class of selective Neurokinin- 1 (NK1) receptor antagonists which, in addition to their use for treating chemotherapy-induced nausea and emesis, also play an important role as therapies for depression and anxiety.
- Description Netupitant is an insurmountable antagonist of the neurokinin-1 (NK1) receptor (Ki = 0.95 nM in CHO cells expressing the human recombinant receptor). It is selective for human NK1 over human NK2 and NK3 and rat NK1 (Kis = >1,500 nM) and over 50 G protein-coupled receptors, monoamine transporters, and ion channels when used in the nanomolar range. Netupitant decreases the maximal response to substance P-induced contractions in isolated guinea pig ileum with long-lasting effects. It also dose-dependently inhibits the substance P-induced scratching, biting, and licking response in mice when used at doses ranging from 1-10 mg/kg and decreases NK agonist-induced foot tapping in gerbils (ID50s = 1.5 mg/kg, i.p., or 0.5 mg/kg, oral). Formulations containing netupitant have been used in the treatment of chemotherapy-induced nausea and vomiting.
- Uses Antiemetic.
- Uses Netupitant is a potent and selective neurokinin-1 receptor (NK1) receptor antagonist. It is achiral and orally active.
- Definition ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid with the secondary amino group of N-methyl-4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin 3-amine; an antiemetic used in combination with palonosetron hydrochloride (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy.
-
Synthesis
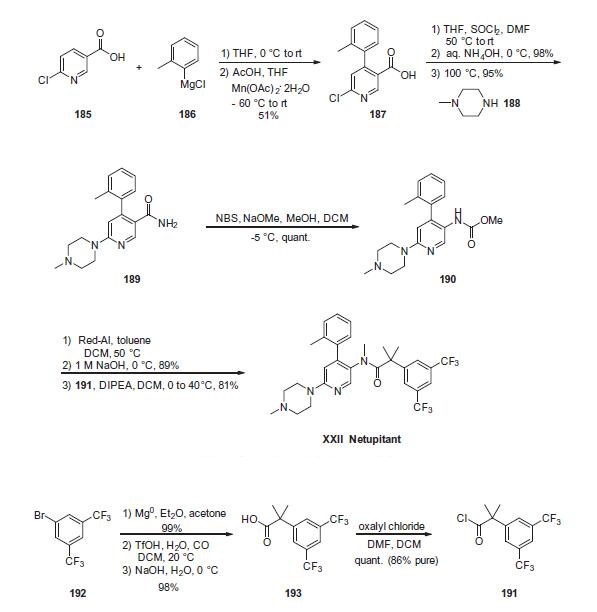
The most likely process-scale synthesis
of netupitant begins with 6-chloronicotinic acid (185). From
185, a one-pot 1,4-Grignard addition/oxidation reaction, developed
to provide an improved route to NK1 receptor antagonists, was
employed for direct installation of the C4-o-tolyl substituent. Using
this procedure, treatment of 6-chloronicotinic acid (185) with otolyl
magnesium chloride and subsequent oxidation with Mn
(OAc)2 in THF/AcOH generated the o-tolyl nicotinic acid intermediate
187 in 51% overall yield. From this intermediate, a one-pot
amide formation could be realized in high yield by conversion of
the acid to the corresponding acyl chloride and addition of NH4OH
(95% yield). Chloride displacement with 1-methyl piperazine under
heating conditions provided intermediate 189 in 95% yield.
Employing Hoffman reaction conditions originally reported by
Senanayake,171 rearrangement of amide 189 with NBS/NaOMe/
MeOH enabled formation of carbamate 190 in quantitative yield.
Reduction of the carbamate with Red-Al provided the desired
mono-methylated amine. To access the final drug target, acylation
of the intermediate methyl amine with 2-(3,5-bis(trifluoromethyl)
phenyl)-2-methylpropanoyl chloride (191) provided the final drug
netupitant (XXII) in 81% yield. In this case, due to the cost of 193,
the acid precursor to 191, and starting materials previously
reported for generating 191/193, as well as issues with isolation
of pure intermediates on scale, a novel route to 191 and 193 was
also developed during this synthesis, beginning with the inexpensive
and readily available bromide 192. This 2-step synthesis of 193 includes Grignard
reagent formation, quenching with acetone to yield the intermediary
tertiary alcohol, and subsequent carbonylation (TfOH, H2O, CO then NaOH/H2O) to provide 2-(3,5-bis(trifluoromethyl)-phenyl)-2-
methylpropanoic acid 193. Finally, conversion of acid 193 to the
acyl chloride with oxalyl chloride in DCM provided the necessary
acyl chloride 191 in quantitative yield (86% purity).
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Preparation Products And Raw materials
Raw materials
Preparation Products
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Suppliers
Global(208)Suppliers
-
Supplier:
Aceschem Inc.
- Tel:+1-817863-6948<br/>+1-(817)863-6948
- Email:sales@aceschem.com
- Country:United States
- ProdList:19632
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
shandong perfect biotechnology co.ltd
- Tel:+86-53169958659<br/>+86-13153181156
- Email:sales@sdperfect.com
- Country:China
- ProdList:294
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Watson Biotechnology Co.,Ltd
- Tel:+86-18186686046<br/>+86-18186686046
- Email:sales01@watsonbiotech.cn
- Country:China
- ProdList:5849
- Advantage:58
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
TianYuan Pharmaceutical CO.,LTD
- Tel:+86-755-23284190 13684996853
- Email:sales@tianpharm.com
- Country:CHINA
- ProdList:304
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Spectrum
290297-26-6, 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamideRelated Search:
- Methyl (6-(4-Methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl)carbaMate 3-PYRIDINAMINE, N-METHYL-4-(2-METHYLPHENYL)-6-(4-METHYL-1-PIPERAZINYL)- 3-PYRIDINECARBOXAMIDE, 4-(2-METHYLPHENYL)-6-(4-METHYL-1-PIPERAZINYL)- N-tert-butyl-6-chloro-4-(o-tolyl)nicotinamide Sulfachoropyrazine sodium Pyrazinamide Indole Furan Pyridoxine Pyrazole Oxazole Pyrrole Pyridine
- 杂质对照品
- 抑制剂
- 合成有机化合物配体
- 医药化工原料
- 医用原料
- 原料药及中间体
- 原料
- 原料药
- 医药原料
- 医药中间体
- 医药原料药
- C30H32F6N4O
- 290297-36-6
- 2-[3,5-双(三氟甲基)苯基]-N,2-二甲基-N-[4-(2-甲基苯基)-6-(4-甲基哌嗪-1-基)吡啶-3-基]丙酰胺
- 网联
- 奈妥匹坦
- 2-(3,5-双(三氟甲基)苯基)-N,2-二甲基-N-(6-(4-甲基哌嗪-1-基)-4-(邻甲苯基)吡啶-3-基)丙酰胺
- 奈妥吡坦/萘妥吡坦
- 网友
- 网状物杂质
- 奈妥匹坦(包括很多杂质)
- 奈妥吡坦
- 萘妥吡坦
- 奈妥吡坦 290297-26-6
- 290297-26-6
- Netupitant
- NetupitantQ: What is Netupitant Q: What is the CAS Number of Netupitant Q: What is the storage condition of Netupitant Q: What are the applications of Netupitant
- 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-meth...
- Benzeneacetamide, N,α,α-trimethyl-N-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoromethyl)-
- NETUPITANT (RO 67-31898)
- 2-[3,5-Bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-y
- Netupitant(CID-6451149)
- CID-6451149
- CID6451149
- CID 6451149
- Netupitan
- Ro 67-31898
- 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-
- BenzeneacetaMide, N,a,a-triMethyl-N-[4-(2-Methylphenyl)-6-(4-Methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoroMethyl)-
- 2-(3,5-bis(trifluoroMethyl)phenyl)-N,2- diMethyl-N-(6-(4-Methylpiperazin-1-yl)-4- o-tolylpyridin-3-yl)propanaMide
- Ro 67-31898/000
- 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
- 2-[3,5-Bis(trifluoromethyl)phenyl]-N-[6-(4-methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl]-N-methylisobutyramide
![2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Structure](https://www.chemicalbook.com/CAS/GIF/290297-26-6.gif)