ChemicalBook > CAS DataBase List > Astemizole
Astemizole
Astemizole
- CAS No.68844-77-9
- Chemical Name:Astemizole
- CBNumber:CB6741448
- Molecular Formula:C28H31FN4O
- Formula Weight:458.57
- MOL File:68844-77-9.mol
Astemizole Property
- Melting point 172.9°C
- Boiling point 627.3±65.0 °C(Predicted)
- Density 1.1587 (estimate)
- storage temp. 2-8°C
- solubility DMSO: >20mg/mL
- pka pKa 4.85(H2O t=25.0 I=0.025) (Uncertain);8.69(H2O t=25.0 I=0.025) (Uncertain)
- form powder
- color Crystals
- Water Solubility It is soluble in DMSO (25 mg/ml ), ethanol (25 mg/ml), chloroform, methanol, and water (partly miscible).
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months.
- FDA UNII 7HU6337315
- ATC code R06AX11
- UNSPSC Code 41121800
- NACRES NA.77
Safety
- Hazard Codes :Xn
- Risk Statements :22-36/37/38
- Safety Statements :26-36
- RIDADR :UN 2811 6.1 / PGII
- WGK Germany :3
- RTECS :DD8968000
- HS Code :2933992600
- Hazardous Substances Data :68844-77-9(Hazardous Substances Data)
- Toxicity :LD50 orl-rat: >2560 mg/kg AIPTAK 251,39,81
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P264-P271-P280-P302+P352-P305+P351+P338
Astemizole Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: A2861
- Product name : Astemizole
- Purity: ≥98% (HPLC)
- Packaging: 10MG
- Price: ₹10857.48
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: A2861
- Product name : Astemizole
- Purity: ≥98% (HPLC)
- Packaging: 50MG
- Price: ₹43354.13
- Updated: 2022/06/14
- Buy: Buy
Astemizole Chemical Properties,Usage,Production
- Uses Astemizole is a histamine H1-receptor antagonist with IC50 of 4.7 nM. Astemizole is also a potent inhibitor of ether à-go-go 1 (Eag1) and Eag-related gene (Erg) potassium channels. Astemizole has antineoplastic and antipruritic effects.
- Description Astemizole belongs to the second-generation class of non-sedating, non-anticholinergic antihistamines. Its non-sedating properties appear to result from its poor penetration of the blood brain barrier. As a result it shows no potentiation of CNS depressants, including alcohol. Its long half-life allows once-daily dosing.
- Chemical Properties Crystalline Solid
- Originator Janssen (Belgium)
- Uses Astemizole is used for preventing and treating severe seasonal and chronic allergic rhinitis, allergic conjunctivitis, hives, Quinke’s edema, other allergic conditions and dermatitis. Synonyms of this drug are hismanal, histazol, and others.
- Uses scabicide
- Uses Nonsedating-type histamine H1-receptor antagonist. Potential for combination therapy with antivancer drugs such as doxorubicin in resistant leukemia. Antihistaminic
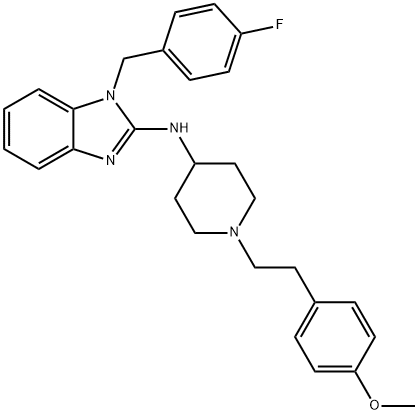
- Definition ChEBI: A piperidine compound having a 2-(4-methoxyphenyl)ethyl group at the 1-position and an N-[(4-fluorobenzyl)benzimidazol-2-yl]amino group at the 4-position.
- Manufacturing Process A mixture of 2.3 parts of 2-(4-methoxyphenyl)ethyl methanesulfonate, 4.9 parts of 1-[(4-fluorophenyl)methyl]-N-(4-piperidinyl)-1H-benzimidazol-2- amine dihydrobromide, 3.2 parts of sodium carbonate, 0.1 part of potassium iodide and 90 parts of N,N-dimethylformamide is stirred overnight at 70°C. The reaction mixture is poured onto water. The product is extracted with methylbenzene. The extract is washed with water, dried, filtered and evaporated. The residue is purified by column-chromatography over silica gel using a mixture of trichloromethane and methanol (98:2 by volume) as eluent. The pure fractions are collected and the eluent is evaporated. The residue is crystallized from 2,2'-oxybispropane, yielding 2.2 parts (48%) of 1- (4-fluorophenylmethyl)-N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1Hbenzimidazol- 2-amine, MP 149.1°C.
- brand name Hismanal (Janssen);Alermizol;Astezol;Astol;Histamanal;Novo-nastizol;EISMANAL.
- Therapeutic Function Antiallergic, Antihistaminic
- World Health Organization (WHO) The first clinically interesting histamine ti-antagonists were introduced in the late forties and early fifties. Several histamine ti-antagonists have a similar cardiac effect to that seen with astemizole and terfenadine. Serious cardiovascular adverse reactions have been reported when used concomitantly with imidazole antifungals and macrolide antibiotics.
- Biological Activity Orally active, potent histamine H 1 antagonist (IC 50 = 4 nM) that displays 20-fold, > 250-fold and > 250-fold selectivity over 5-HT, dopamine and muscarinic acetylcholine receptors respectively. Exhibits antimalarial activity in multidrug resistant strains in vitro (IC 50 = 227 - 734 nM). Also potent hERG K + channel blocker (IC 50 = 0.9 nM) that displays cardiotoxicity in vivo .
- Biochem/physiol Actions Astermizole is a potent hERG potassium channel blocker (IC50 of 0.9 nM) and may used as a pharmacological chaperone to correct folding defects and restore protein function for some mutated forms of hERG channels. It has also been studied for treatment of malaria, hERG and hEAG channel function in cancer and as a second generation antihistamine H-1 antagonist.
- Safety Profile Poison by subcutaneous andintravenous routes. Moderately toxic by ingestion. Humansystemic effects by ingestion: arrhythmias, coma, nauseaor vomiting, somnolence. When heated to decompositionit emits toxic fumes of F?? and NOx.
-
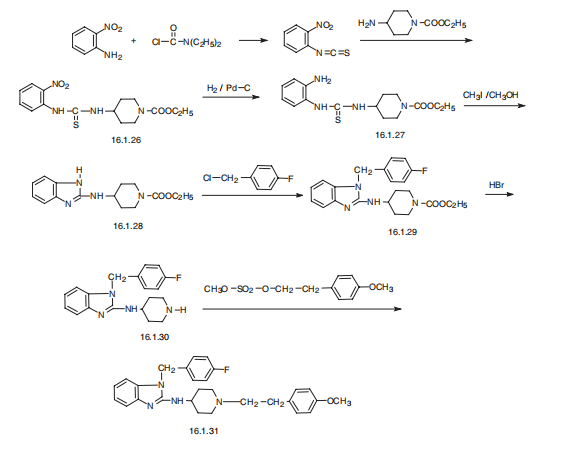
Synthesis
Astemizole, 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl) ethyl]-4-
piperidinyl]-benzimidazol-2-amine (16.1.31), is synthesized in a multi-stage synthesis from
1-carbethoxy-4-aminopiperidine and 2-nitroisothiocyanobenzol, from which a derivative of
thiourea (16.1.26) is synthesized upon their reaction. The nitro group of the product is
reduced and the further S-methoxided. In reaction conditions intermolecular cyclization into
a derivative of benimidazol, N-[1-[2-(4-carethoxy)]-4-piperidinyl]benzimidazol-2-amine
(16.1.28) occurs. The obtained aminobenzimidazole derivative is alkylated with 4-fluorobenzylchoride
into 1-[(flurophenyl)methyl]-N-[1-[2-(4-carethoxy)]-4-piperidinyl] benzimidazol-
2-amine (16.1.29). The carbethoxyl group of the resulting compound (16.1.29) is
hydrolyzed by hydrobromic acid, forming a non-substituted on the nitrogen atom derivative
of piperidine (16.1.30), the alkylation of which with 2-(4-methoxyphenyl)ethylmetanesulfonate
leads to the formation of astemizole (16.1.31).
- storage Store at +4°C
- References 1) Richards et al. (1984), Astemizole. A review of its pharmacodynamic properties and therapeutic efficacy; Drugs, 28 38 2) Laduron et al. (1982), In vitro and in vivo binding characteristics of a new long-acting histamine H1 antagonist, astemizole; Mol. Pharmacol., 21 294
Astemizole Preparation Products And Raw materials
Raw materials
Preparation Products
Global(191)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21629
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7832
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15350
- Advantage:58
-
Supplier:
Shanghai kingmorn biotechnology Co.,Ltd.
- Tel:021-62200788<br/>15000569291
- Email:2199306478@qq.com
- Country:CHINA
- ProdList:99
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
InvivoChem
- Tel:+1-708-310-1919<br/>+1-13798911105
- Email:sales@invivochem.cn
- Country:United States
- ProdList:6391
- Advantage:58
-
Supplier:
LEAP CHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:24727
- Advantage:58
68844-77-9, AstemizoleRelated Search:
- Cisapride Benzene Astemizole 1-BENZYL-1H-BENZOIMIDAZOL-2-YLAMINE 2-AMINO-1-METHYLBENZIMIDAZOLE norastemizole 2-(AMINOMETHYL)-1-METHYLIMIDAZOLE 1-ETHYL-1H-BENZOIMIDAZOL-2-YLAMINE 1-PHENETHYL-PIPERIDIN-4-YLAMINE 4-AMINO-1-(1-PROPYL)-PIPERIDINE 1-(4-METHOXYPHENETHYL)-4-AMINOPIPERIDINE 1-BENZYL-1H-IMIDAZOL-2-YLAMINE O-Desmethyl Astemizole 2-AMINO-1-((4-FLUOROPHENYL)METHYL)BENZI& DESMETHYL ASTEMIZOLE astemizole tetrachlorocuprate(II) DESMETHYL ASTEMIZOLE HYDROCHLORIDE ASTEMIZOLE, [METHYL-3H]-
- Histamine receptor
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Aromatics
- Amines
- ASCABIOL
- 药靶配体
- FDA批准的配体
- 原料药
- 抑制剂
- 生化试剂
- 中药对照品
- 标准品
- 抗组胺药
- 药物
- 组胺H1受体拮抗剂
- 合成
- 小分子抑制剂
- Benzimidazoles
- Heterocyclic Building Blocks
- Building Blocks
- C28H31FN4O
- C28H33FN4O
- 68844-79-9
- 阿司咪唑,10 MM DMSO 溶液
- ASTEMIZOLE,HISTAMINE H1 RECEPTOR拮抗剂AND HERG K+ CHANNEL BLOCKER
- 甲醇中阿司咪唑
- 1-(4-氟苄基)-N-(1-(4-甲氧基苯乙基)哌啶-4-基)-1H-苯并[D]咪唑-2-胺
- 甲醇中阿司咪唑标准溶液
- 息斯敏对照品
- 息斯敏标准品
- ASTEMIZOLE[干冰运输]
- 阿司咪唑 USP标准品
- 阿司咪唑
- 1-(4-氟苄基)-2-(1-[4-甲氧基苯乙基]哌啶-4-基)氨基苯并咪唑
- 息斯敏
- 1-[(4-氟苯基)甲基]-N-[1-[2-(4-甲氧苯基)乙基]-4-哌啶基]-1H-苯并咪唑-2-胺
- 阿司咪唑杂质
- 阿司咪唑溶液,100PPM
- 68844-77-9
- Astemizole, 10 mM in DMSO
- Astemizole, histamine H1 receptor antagonist and hERG K+ channel blocker
- Astemizole(R43512)
- 1-(4-Fluorobenzyl)-N-(1-(4-methoxyphenethyl)piperidin-4-yl)-1H-benzo[d]imidazol-2-amine
- 1H-Benzimidazol-2-amine, 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-
- Astemizole USP/EP/BP
- AstemizoleQ: What is Astemizole Q: What is the CAS Number of Astemizole Q: What is the storage condition of Astemizole Q: What are the applications of Astemizole
- 68844-77-9 Astemizole
- Astemizole (1044301)
- Astemizole (200 mg)
- 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl]benzimidazol-2-amine
- [1-(4-fluorobenzyl)benzimidazol-2-yl]-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidyl]amine
- r42512
- paralergin
- novo-mastizola
- metodih
- laridal