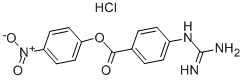
ChemicalBook > CAS DataBase List > NPGB
NPGB
NPGB
- CAS No.19135-17-2
- Chemical Name:NPGB
- CBNumber:CB6287205
- Molecular Formula:C14H13ClN4O4
- Formula Weight:336.73
- MOL File:19135-17-2.mol
NPGB Property
- Melting point 241-243 °C
- storage temp. -20°C
- solubility formic acid: soluble 49.00-51.00mg/mL
- form Solid
- color White to off-white
- BRN 8024554
- UNSPSC Code 12352204
- NACRES NA.32
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H318
- Precautionary statements P280-P305+P351+P338
NPGB Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: N8010
- Product name : 4-Nitrophenyl 4-guanidinobenzoate hydrochloride
- Purity: protease inhibitor and substrate
- Packaging: 500MG
- Price: ₹22570.13
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: N8010
- Product name : 4-Nitrophenyl 4-guanidinobenzoate hydrochloride
- Purity: protease inhibitor and substrate
- Packaging: 1G
- Price: ₹37238
- Updated: 2022/06/14
- Buy: Buy
NPGB Chemical Properties,Usage,Production
-
Uses
4-Nitrophenyl 4-guanidinobenzoate hydrochloride has been used:
- as a substrate for trypsin for active site titration experiments
- for pre-treating of mosquito eggs in the interplasmid transposition assay
- as a component of isotonic buffer to moisten filter paper for mosquito embryo collection
- General Description 4-Nitrophenyl 4-guanidinobenzoate hydrochloride (pNPGB) is a trypsin substrate. It is more water-soluble than 4-methylumbelliferyl 4-guanidinobenzoate and is preferred in spectrophotometric assay.
- Biochem/physiol Actions 4-Nitrophenyl 4-guanidinobenzoate hydrochloride (pNPGB) inhibits phenoloxidase enzyme and delays embryo chorionic membrane hardening.
-
References
[1] BHUMI PATEL . L. donovani XPRT: Molecular characterization and evaluation of inhibitors[J]. Biochimica et biophysica acta. Proteins and proteomics, 2018, 1866 3: Pages 426-441. DOI:10.1016/j.bbapap.2017.12.002.
[2] FRANK S. STEVEN Margaret M G. Studies on the Molecular Mechanism of Mersalyl and 4-Aminophenylmercuric Acetate Re-activation of Trypsin-Thiol Complexes[J]. The FEBS journal, 1980, 109 2: 567-573. DOI:10.1111/j.1432-1033.1980.tb04829.x.
[3] YING-YING HE . Isolation, expression and characterization of a novel dual serine protease inhibitor, OH-TCI, from king cobra venom[J]. Peptides, 2008, 29 10: Pages 1692-1699. DOI:10.1016/j.peptides.2008.05.025.
[4] J.A. SCHIFFERLI G. S. A simple two-step procedure for the preparation of the first component of human complement (C1) in its native form[J]. Journal of immunological methods, 1985, 76 2: Pages 283-288. DOI:10.1016/0022-1759(85)90305-9.
[5] J. M. KAMINSKI. Synthesis and inhibition of human acrosin and trypsin and acute toxicity of aryl 4-guanidinobenzoates[J]. Journal of Medicinal Chemistry, 1986, 29 4: 514-519. DOI:10.1021/jm00154a015.
NPGB Preparation Products And Raw materials
Raw materials
Preparation Products
Global(18)Suppliers
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: ChemShuttle, Inc.
- Tel:0150-83588313-811<br/>18800520310
- Email:sales@chemshuttle.com
- Country:China
- ProdList:2999
- Advantage:62
- Supplier: Sigma-Aldrich
- Tel:021-61415566<br/>800-8193336
- Email:orderCN@merckgroup.com
- Country:China
- ProdList:51456
- Advantage:80
- Supplier: Beijing Haite lesk Technology Co., Ltd
- Tel:15210893940;<br/>15116916250
- Email:chemistryliu@163.com
- Country:China
- ProdList:7327
- Advantage:58
- Supplier: Energy Chemical
- Tel:021-58432009<br/>400-005-6266
- Email:marketing@energy-chemical.com
- Country:China
- ProdList:44918
- Advantage:58
- Supplier: Shaanxi Dideu Newmaterial Co., Ltd.
- Tel:029-87576359<br/>15353716720
- Email:1039@dideu.com
- Country:China
- ProdList:9937
- Advantage:58
- Supplier: Shanghai Yingxin laboratory equipment Co., Ltd
- Tel:021-021-59178156<br/>15300768757
- Email:359382369@qq.com
- Country:China
- ProdList:10674
- Advantage:58
- Supplier: Shanghai Guchen Biotechnology Co., LTD
- Tel:021-34675735<br/>19147740836
- Email:1986399151@qq.com
- Country:China
- ProdList:9844
- Advantage:58
- Supplier: Merck KGaA
- Tel:21-20338288
- Email:ordercn@merckgroup.com
- Country:China
- ProdList:292394
- Advantage:58
19135-17-2, NPGBRelated Search:
- 1-phenylguanidine 4-(Methylamino)benzoic acid 4-AMINOBENZOIC ACID HCL 4-BENZYLOXYANILINE 4-Guanidinobenzoic acid hydrochloride METHYL 4-METHYLAMINOBENZOATE 1-BENZYLOXY-4-NITROBENZENE 4-NITROPHENYL BENZOATE 4-Benzyloxyaniline hydrochloride Phenyl benzoate PHENYL 4-AMINOBENZOATE N-P-TOLYL-GUANIDINE HYDROCHLORIDE P-PHENETIDINE HYDROCHLORIDE N-P-TOLYL-GUANIDINE NPGB METHYL 4-AMINOBENZOATE HYDROCHLORIDE 4-(PHENOXYMETHYL)ANILINE 4-nitrophenyl 4'-guanidinobenzoate
- Biochemicals and Reagents
- BioChemical
- Activity
- Enzyme Substrates
- Enzymes, Inhibitors, and Substrates
- Chromogenic
- Substrates by Enzyme
- C14H12N4O4HCl
- 4-胍基苯甲酸4-硝基苯酯盐酸盐
- 19135-17-2
- 4-Nitrophenyl 4-guanidinobenzoate hydrochloride protease inhibitor and substrate
- (4-nitrophenyl)4-(diaminomethylideneamino)benzoate,hydrochloride
- 4-Guanidinobenzoic acid 4-nitrophenylester hydrochloride, NPGB, pNPGB
- Benzoic acid, 4-(aminoiminomethyl)amino-, 4-nitrophenyl ester, monohydrochloride
- p-Nitrophenyl-p'-guanidinobenzoatehydrochloridecrystalline
- p-nitrophenyl-p'-guanidinobenzoateHClcrystalline
- 4-NITROPHENYL 4-GUANIDINOBENZOATE &
- P-NITROPHENYL P'-GUANIDINOBENZOATE HCL
- 4-nitrophenyl 4-[(aminoiminomethyl)amino]benzoate monohydrochloride
- Nsc163801
- P'-GUANIDINOBENZOIC ACID P-NITROPHENYL ESTER HYDROCHLORIDE
- P-NITROPHENYL-P-GUANIDINOBENZOATE HYDROCHLORIDE
- P-NITROPHENYL P'-GUANIDINOBENZOATE HYDROCHLORIDE
- PNPGB
- NPGB
- 4-NITROPHENYL 4-GUANIDINOBENZOATE HYDROCHLORIDE
- 4-GUANIDINOBENZOIC ACID 4-NITROPHENYL-ESTER HYDROCHLORIDE