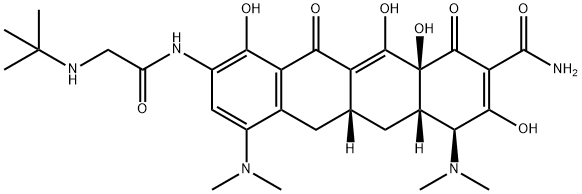
Tigecycline
- CAS No.220620-09-7
- Chemical Name:Tigecycline
- CBNumber:CB6248215
- Molecular Formula:C29H39N5O8
- Formula Weight:585.65
- MOL File:220620-09-7.mol
- Melting point 164-166°C
- Boiling point 890.9±65.0 °C(Predicted)
- Density 1.45±0.1 g/cm3(Predicted)
- storage temp. Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
- solubility Soluble in DMSO (up to at least 25 mg/ml).
- pka 4.50±1.00(Predicted)
- form Orange powder
- color Orange
- Merck 14,9432
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month.
- InChIKey FPZLLRFZJZRHSY-HJYUBDRYSA-N
- SMILES C1(=O)[C@]2(O)[C@@]([H])(C[C@@]3([H])C(=C2O)C(=O)C2=C(C(N(C)C)=CC(NC(CNC(C)(C)C)=O)=C2O)C3)[C@H](N(C)C)C(O)=C1C(N)=O
- CAS DataBase Reference 220620-09-7(CAS DataBase Reference)
- FDA UNII 70JE2N95KR
- NCI Drug Dictionary tigecycline
- ATC code J01AA12
- UNSPSC Code 41116107
- NACRES NA.24
- Safety Statements :24/25
- RIDADR :3077
- RTECS :QI7619500
- HazardClass :9
- PackingGroup :III
- HS Code :29419090
- Hazardous Substances Data :220620-09-7(Hazardous Substances Data)
-
NFPA 704:
0 2 0
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H319-H360D-H372-H410
- Precautionary statements P202-P260-P264-P273-P305+P351+P338-P308+P313
- Brand: Sigma-Aldrich(India)
- Product number: PHR2591
- Product name : Tigecycline
- Purity: certified reference material, pharmaceutical secondary standard
- Packaging: 500MG
- Price: ₹22342.8
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T3589
- Product name : Tigecycline
- Purity:
- Packaging: 100MG
- Price: ₹7200
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T3589
- Product name : Tigecycline
- Purity:
- Packaging: 500MG
- Price: ₹14600
- Updated: 2022/05/26
- Buy: Buy
Tigecycline Chemical Properties,Usage,Production
-
Indications and Usage
Tigecycline is also called 9-tert-glycylaminomycetine or diclofenac, and it is a new type of venous injection antibiotic with broad-spectrum activities. It is a type of 9-tert-glycylaminomycetine derivative and is the first glycylcine antibiotic.
Tigecycline can serve as a second option after failed first-line treatment for multi-drug resistant bacteria, and it is also a new treatment option for patients who are allergic to penicillin or intolerable to other drugs. It can treat patients 18 years old or above with complex skin and skin structure infections or complex abdominal infections such as complex appendicitis, burn infections, abdominal abscesses, deep soft tissue infections, and ulcer infections. - Mechanisms of Action Tigecycline’s mechanisms of action are similar to those of tetracycline antibiotics, which are binding with bacterial 30S ribosomes to prevent transfer RNA from entering, making it impossible for amino acids to form peptide chains, thus preventing bacterial protein synthesis and limiting bacterial growth. However, tigecycline’s ability to bind with ribosomes is 5 times that of other tetracycline antibiotics, which means that tetracycline’s anti-drug resistance ability is stronger. Tigecycline’s structure is similar to that of minocycline, but tigecycline’s antibacterial activity is much stronger, and bacteria are less likely to develop resistance to it compared to other tetracycline drugs, and it can also act on the methicillin-resistant Staphylococcus aureus. Tigecycline’s antifungal spectrum includes gram-positive bacteria, gram-negative bacteria and anaerobic bacteria. In vitro experiments and clinical trials showed that tigecycline is sensitive to some aerobic gram-negative bacteria (such as Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae and Klebsiella pneumoniae, Acinetobacter baumannii, Aeromonas hydrophila, Citrobacter Enterobacteriaceae, hemorrhagic Pasteurella, Serratia marcescens, and Stenotrophomonas maltophilia). Pseudomonas auruginosa is resistant to tigecycline.
- Adverse reaction The most common adverse effects are nausea and vomiting, which usually happens in the first 1-2 days of treatment and are mild to moderate in intensity. In a positive drug control clinical trial, 35% percent of complex skin and skin structure infection patients using tigecycline experienced nausea, and 20% experienced vomiting; vancomycin/aztreonam use caused 8.9% nausea and 4.2% vomiting. 25.3% of complex abdominal infection patients using tigecycline experienced nausea, and 19.5% experienced vomiting; vancomycin/aztreonam caused 20.5% nausea and 15.3% vomiting.
- Description Tigecycline is a broad-spectrum glycylcycline antibiotic that binds to the bacterial 30S ribosome, blocking the entry of transfer RNA, which halts protein synthesis and inhibits bacterial growth. It is active against a panel of 1,924 European clinical bacterial isolates including S. aureus, S. epidermidis, S. pneumoniae, E. faecalis, E. faecium, E. coli, K. pneumoniae, P. aeruginosa, and P. mirabilis strains (MICs = <1-32 μg/ml). In vivo, tigecycline (6.25 mg/kg twice daily for 5 days) decreases levels of C. difficile cytotoxin activity and spore formation in cecum and colon in a mouse model of C. difficile infection. Formulations containing tigecycline have been used in the treatment of a variety of bacterial infections.
-
Description
The emergence of drug-resistant bacteria has diminished the clinical utility of the tetracyclines. Research to circumvent the efflux and ribosomal protection mechanisms of bacteria has led to the development of the glycylcyclines. Tigecycline is the first glycylcycline antibiotic to launch for the parenteral treatment of baterial infection, including complicated intra-abdominal and skin infections. Its mechanism of action involves inhibiting protein translation in bacteria by binding to the 30S ribosomal subunit and blocking entry of amino-acyl tRNA molecules into the A site of the ribosome to effectively prevent incorporation of amino acid residues into elongating peptide chains. Presumably, ribosomal protection proteins are ineffective against tigecycline due to its higher affinity for ribosomal binding compared to tetracyclines (approximately 16-fold). In addition, tigecycline may be resistant to efflux mechanisms by either their inability to translocate it across the cytoplasmic membrane due to steric complications or simply by their failure to recognize the molecule.
- Chemical Properties Orange Solid
- Originator Wyeth (US)
- Uses A broad spectrum glycylcycline antibiotic
- Uses antineoplastic
- Uses A glycylcycline antibiotic, used to treat infection by drug resistant bacteria such as Staphylococcus aureus (Staph aureus) and Acinetobacter baumannii.
- Uses Tigecycline is a semi-synthetic tetracycline prepared by the introduction of a tert-butylaminoacetamido group into a previously unexplored and un-substituted region of existing tetracyclines. Like other tetracyclines, tigecycline acts by reversibly binding to the 30S ribosomal subunit and inhibits protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site. The enhanced activity can be attributed to stronger binding affinity, thus minimising the impact of existing mechanisms of resistance. Tigecycline is regarded as the first of a new class of glycylcyline antibiotics. Critical comparison to the tetracycline class appears to be lacking in the literature.
- Definition ChEBI: Tetracycline in which the hydroxy group at position 5 and the methyl group at position 6 are replaced by hydrogen, and with a dimethylamino substituent and an (N-tert-butylglycyl)amino substituent at positions 7 and 9, respe tively. A glycylcycline antibiotic, it has activity against a broad range of Gram-positive and Gram-negative bacteria, including tetracycline-resistant organisms. It is used for the intravenous treatment of complicated skin and skin structure infections ca sed by susceptible organisms.
- brand name Tygacil
-
Antimicrobial activity
It is as potent as, or more potent than,
earlier tetracyclines and activity is retained against strains
expressing acquired tetracycline resistance determinants. It
displays better activity than tetracycline, doxycycline or
minocycline against Streptococcus spp. and against Enterococcus
faecalis and E. faecium. Among Gram-negative organisms it
displays improved activity against Citrobacter freundii,
Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae,
Salmonella spp., Serratia marcescens and Shigella spp. The
spectrum includes rapidly growing mycobacteria. Ps. aeruginosa,
Pr. mirabilis, other Proteus spp. and some strains of
Corynebacterium jeikeium are resistant. Activity against strains
expressing acquired resistance to earlier tetracyclines is
attributed to failure of the MFS efflux pumps to recognize
tigecycline, and to a novel mechanism of ribosome binding
that permits tigecycline to overcome ribosomal protection
mechanisms.
Comparative susceptibility data for some atypical pathogens are not available. However, in common with earlier tetracyclines, it is active against Chlamydophila and Mycoplasma spp. and rapidly growing Mycobacteria spp. It is less active than minocycline or tetracycline against U. urealyticum. -
General Description
Tigecycline (Tygacil) is a first-in-class (a glycylcycline) intravenousantibiotic that was designed to circumvent manyimportant bacterial resistance mechanisms. It is not affectedby resistance mechanisms such as ribosomal protection, effluxpumps, target site modifications, β-lactamases, or DNAgyrase mutations. Tigecycline binds to the 30S ribosomalsubunit and blocks peptide synthesis. The glycylcyclinesbind to the ribosome with five times the affinity of commontetracyclines. Tigecycline also possesses a novel mechanismof action, interfering with the mechanism of ribosomal protectionproteins. Tigecycline, unlike common tetracyclines,is not expelled from the bacterial cell by efflux pumpingprocesses.
Tigecycline is recommended for the treatment of complicatedskin and skin structure infections caused by E. coli,E. faecalis (vancomycin-susceptible isolates), S. aureus(methicillin-susceptible and methicillin-resistant isolates),S. pyogenes, and B. fragilis among others. Tigecycline is alsoindicated for complicated intra-abdominal infections causedby strains of Clostridium, Enterobacter, Klebsiella, andBacteroides. To reduce the development of resistance to tigecycline,it is recommended that this antibiotic be used onlyfor those infections caused by proven susceptible bacteria.Glycylcyclines are structurally similar to tetracyclines,and appear to have similar adverse effects. These mayinclude photosensitivity, pancreatitis, and pseudotumorcerebri. Nausea and vomiting have been reported. - Pharmaceutical Applications 9-T-butylglycylamido-minocycline. A compound of the glycylcycline class available as a powder for intravenous infusion.
-
Pharmacokinetics
Cmax 100 mg intravenous infusion (1 h): 0.85–1 mg/L
Plasma half-life: 37–67 h
Volume of distribution: 7–10 L/kg
Plasma protein binding: 68%
Distribution and excretion
It is widely distributed and is concentrated in the gallbladder, colon and lung. The volume of distribution is dose related and variable, but is generally greater than that of older tetracyclines. CSF penetration is poor. Tigecycline is excreted in the feces and urine predominantly as the unchanged molecule. The elimination half-life is long (37–67 h). Tigecycline clearance is decreased by 20% in patients with renal failure. No dosage adjustments are apparently necessary for tigecycline in patients with renal impairment. -
Clinical Use
Complicated skin and skin structure infections
Complicated intra-abdominal infections
Community-acquired bacterial pneumonia
Recommended principally for the treatment of infections with multiresistant organisms. - Side effects Side effects typical of the group, including nausea, vomiting, diarrhea and headache, occur. Occasional cases of pancreatitis, hypoproteinemia, antibiotic-associated colitis and thrombocytopenia have also been reported.
-
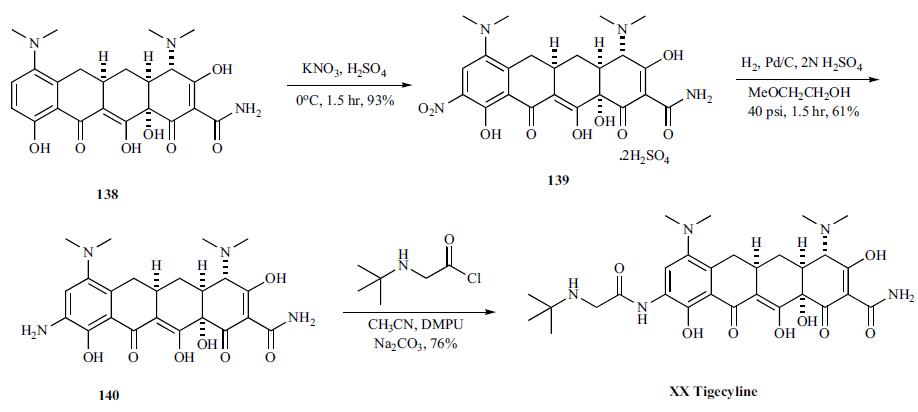
Synthesis
It
does not require dosage adjustment in patients with impaired
renal function and is conveniently dosed every 12 hours.
Synthesis of tigecycline started with nitration
of 138 with potassium nitrate and concentrated sulfuric
acid to give 9-nitro derivative 139 in 93 % yield as disulfate
salt, which was hydrogenated over Pd/C in 2-methoxyethanol/
2N sulfuric acid at 40 psi to provide 9-aminominocycline
(140). Finally, 9-aminominocycline (140) is acylated directly
with N-tert-butylglycyl chloride in a 1:5 mixture of acetonitrile
and N, N-dimethylpropyleneurea (DMPU) with anhydrous
sodium carbonate to give tigecycline (XX).
- in vitro tigecycline exihibited good in vitro activities. the range of mic90s was 0.12-0.5 μg/ml for vancomycin-susceptible and -resistant strains of enterococcus faecalis and enterococcus faecium [2]. tigecyclinewas concentrated in cells and eliminated primarily via biliary excretion. diminished renal function didn’t significantly alter its systemic clearance. tigecycline didn’t interfere with common cytochrome p450 enzymes, making pharmacokinetic drug interactions uncommon [3].the tissue penetration of tigecycline was excellent and the compound showed equivalence to imipenem/cilastatin in intra-abdominal infection and to vancomycin plus aztreonam in skin and skin structure infection [4].
- in vivo in an intraperitoneal systemic murine infection model, tigecycline exihibited in vivo activities against gisa, methicillin-susceptible s. aureus and methicillin-resistant s. aureus strains [2]. tigecycline and daptomycin showed similar in vivo efficacies against infections caused by the mssa strain (strain gc 4543) with the ed50s of 0.12 and 0.24 mg/kg, respectively. the ed50s of tigecycline was 0.72 mg/kg [2].
-
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhanced anticoagulant effect of coumarins.
Oestrogens: possibly reduced contraceptive effects of oestrogens (risk probably small). -
Metabolism
Tigecycline is not thought to be extensively metabolised,
although some trace metabolites have been identified
including a glucuronide, an N-acetyl metabolite, and a
tigecycline epimer. Tigecycline is primarily eliminated
(about 60
%) via biliary excretion of unchanged drug and some metabolites. - References 1) Greer (2006)?Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics; Proc. (Bayl. Univ. Med. Cent.)?19?155 2) Peterson (2008)?A review of tigecycline – the first glycylcycline; Int. J. Antimicrob. Agents?32 Suppl 4?S215 3) Skrtic?et al.?(2011)?Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia; Cancer Cell?20?674 4) Jia?et al.?(2016)?Tigecyclin targets nonsmall cell lung cancer through inhibition of mitochondrial function; Fundam. Clin. Pharmacol.?30?297 5) Hu?et al.?(2016)?Antibiotic drug tigecycline inhibits melanoma progression and metastasis in a p21CIP1/Waf1-dependent manner; Oncotarget?7?3171 6) D’Andrea?et al.?(2016)?The mitochondrial translational machinery as a therapeutic target in Myc-driven lymphomas.; Oncotarget?7?72415 7) Chen?et al.?(2019)?Inhibition of mitochondrial translation selectively targets osteosarcoma; Biochem. Biophys. Res. Commun. 515 9
-
Supplier:
Senova Technology Co. Ltd.
- Tel:+86-0755-86703119<br/>+8618503098836
- Email:info@senovatech.com
- Country:China
- ProdList:351
- Advantage:58
-
Supplier:
Beijing Hope Pharmaceutical Co., Ltd.
- Tel:+86-010-67886402<br/>+8613611125266
- Email:market@hopelife.cn
- Country:China
- ProdList:72
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Nanjing Gold Pharmaceutical Technology Co. Ltd.
- Tel:025-84209270 15906146951
- Email:
- Country:CHINA
- ProdList:115
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Lianyungang happen teng technology co., LTD
- Tel:15950718863
- Email:wang666xt@163.com
- Country:CHINA
- ProdList:295
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
Related articles
- 4'-CHLOROACETANILIDE Minocycline 3-Dimethylaminopropylamine Acetaminophen Minocycline hydrochloride DIACETAMIDE tert-Butanol Thioacetamide Cyclosporin A Chloroacetamide Acetamide Voriconazole Tianeptine Tiagabine hydrochloride N,N-Dimethylacetamide Tigecycline Impurity 1 Tigecycline Impurity 2 Tigecycline Pentacyclic Analog
- 220620-09-7
- BDO
- Antibacterial
- API
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Chiral Reagents
- Amines
- pharmaceutical intermediate
- 高纯试剂
- 四环素
- 抑制剂
- 生化试剂
- 化学原料
- 试剂
- 标准品
- 临床检测标准物质
- 小分子
- 试剂盒-细胞分析试剂盒
- 杂质对照品
- 通用生化试剂-抗生素
- 日用化学品
- 化学试剂
- 精细化工原料
- 抗生素类
- 医用原料
- 药物杂质及中间体
- 产品
- 医药抗生素
- 中药对照品
- 对照品-杂质对照品
- 医药化工类
- 精细化工
- 原料药
- 医药原料药
- 医药原料
- 抗生素
- 微生物代谢物
- 小分子抑制剂
- 原料
- Antibiotics抗生素类
- 科研试剂
- C29H39N5O8
- 20620-09-7
- 替加环素,10 MM DMSO 溶液
- TIGECYCLINE,GLYCYLCYCLINE ANTIBIOTIC
- 替加环素检测标准品
- [D9]-替加环素/精确称量
- 优选]替加环素
- 优选]TIGECYCLINE
- 泰格环素
- 替加环素(对照品)
- 2H9]-替加环素
- 替加环素/9-叔丁基甘氨酰氨基米诺环素
- 9-叔丁基甘氨酰氨基米诺环素
- AB-PINACA N-戊酸代谢产物
- (4S,4AS,5AR,12AS)-4,7-双(二甲氨基)-9-[(叔丁基氨基)乙酰胺基]-3,10,12,12A-四羟基-1,11-二氧代-1,4,4A,5,5A,6,11,12A-八氢并四苯-2-甲酰胺/2-并四苯甲酰胺, 4,7-双(二甲胺基)-9-[[2-[(1,1-二甲基乙基)氨基]乙酰基]氨基]-1,4,4A,5,5A,6,11,12A-八氢-3,10,12,12A-四羟基-1,11-二氧代-, (4S,4AS,5AR,12AS)-
- (4S,4AS,5AR,12AS)-9-(2-(叔丁基氨基)乙酰氨基)-4,7-双(二甲基氨基)-3,10,12,12A-四羟基-1,11-二氧代-1,4,4A,5,5A,6,11,12A-八氢并四苯-2-甲酰胺