ChemicalBook > CAS DataBase List > TETRAIODOPHTHALIC ANHYDRIDE
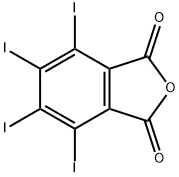
TETRAIODOPHTHALIC ANHYDRIDE
TETRAIODOPHTHALIC ANHYDRIDE
- CAS No.632-80-4
- Chemical Name:TETRAIODOPHTHALIC ANHYDRIDE
- CBNumber:CB6193948
- Molecular Formula:C8I4O3
- Formula Weight:651.7
- MOL File:632-80-4.mol
TETRAIODOPHTHALIC ANHYDRIDE Property
- Melting point 327.5°C
- Boiling point 584.2±50.0 °C(Predicted)
- Density 3.1538 (estimate)
- EPA Substance Registry System 1,3-Isobenzofurandione, 4,5,6,7-tetraiodo- (632-80-4)
Safety
- HazardClass :IRRITANT
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P280-P305+P351+P338
TETRAIODOPHTHALIC ANHYDRIDE Chemical Properties,Usage,Production
-
Preparation
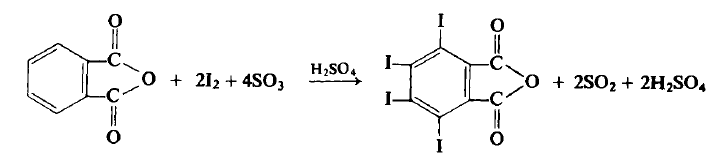
CAUTION: This reaction should be carried out in a well-ventilated hood. <br/>To a flask equipped with a mechanical stirrer and an air condenser topped with a tube leading to a gas trap is added 74.0 gm (0.5 mole) of phthalic anhydride, 162 gm (0.638 mole) of iodine, and 300 ml of 60% fuming sulfuric acid (1.84 moles). The flask is gently heated to 45-50°C, at which point the reaction commences. If the reaction becomes too vigorous it may be necessary to use a ice bath to lower the temperature to 40-50°C. The reaction mixture is eventually (4 hr) heated up to 65°C until all visible reaction has ceased. The reaction mixture is cooled to 10-20°C and an additional 81.0 gm (0.318 mole) of iodine is added and the reaction again slowly heated up to 65°C (1?hr) and again when the reaction ceases it is cooled. Another 27.0 gm (0.107 mole) of iodine is added and the reaction is again heated up to 65°C (1 hr). The flask is heated with an oil bath to a bath temperature of 175-180°C, at which point the sulfur trioxide and iodine fumes evolve. After about 2 hr, when the gaseous evolution ceases, the flask is cooled to about 60°C and the mxiture then poured into a beaker of water. The contents are allowed to stand overnight at room temperature, filtered, washed with two 50-ml portions of cone, sulfuric acid and then with three 100 ml portions of water. The light yellow crystalline product is put into a beaker containing 1 liter of water and 10 gm of sodium bisulfite in order to remove the last traces of free iodine. The aqueous solution is decanted, the product washed five times with ? liter of water, washed twice with 100 ml of acetone and then dried at 60°C to afford 260-268 gm (80-82%), m.p. 327-328°C.
TETRAIODOPHTHALIC ANHYDRIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(59)Suppliers
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
Finetech Industry Limited
- Tel:+86-27-87465837<br/>+8618971612321
- Email:info@finetechnology-ind.com
- Country:China
- ProdList:9642
- Advantage:58
-
Supplier:
Nextpeptide Inc
- Tel:+86-0571-81612335<br/>+8613336028439
- Email:sales@nextpeptide.com
- Country:China
- ProdList:19908
- Advantage:58
-
Supplier:
RR Scientific LLC
- Tel: +86-13917743231
- Email:sales@rrscientific.com
- Country:United States
- ProdList:878
- Advantage:58
-
Supplier:
LEAPCHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:43340
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
SHANGHAI KEAN TECHNOLOGY CO., LTD.
- Tel: +8613817748580
- Email:cooperation@kean-chem.com
- Country:China
- ProdList:40066
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39894
- Advantage:58
632-80-4, TETRAIODOPHTHALIC ANHYDRIDERelated Search:
- 2-Iodobenzoic acid Methyl 2-iodobenzoate TETRAIODOPHTHALIC ANHYDRIDE 3,4,5-Triiodobenzyl alcohol Methyl 4-iodobenzoate 4-Iodobenzyl alcohol 1,2-Diiodobenzene 2,3,5-Triiodobenzoic acid 3,4-Diiodobenzoic acid 1-Iodo-2,3-dimethylbenzene 3-Iodobenzoic acid 3,4,5-Triiodobenzoic acid Methyl 3-iodobenzoate 3,5-Diiodobenzoic acid 3-Iodo-2-methylbenzoic acid 5-Iodo-2-methylbenzoic acid 4-Iodobenzoic acid 1,4-Diiodobenzene
- 合成材料中间体
- C8I4O3
- I4C6CO2O
- 4,5,6,7-TETRAIODOISOBENZOFURAN-1,4-DIONE
- 四碘苯酐
- 四碘邻苯二甲酸酐
- 四碘酞[酸]酐
- 632-80-4
- 3,4,5,6-Tetraiodophthalic anhydride
- TETRAIODOPHTHALIC ANHYDRIDE
- Phthalicanhydride,tetraiodo-
- 4,5,6,7-tetraiodo-3-isobenzofurandione
- 1,3-Isobenzofurandione,4,5,6,7-tetraiodo-
- 4,5,6,7-tetraiodo-1,3-Isobenzofurandione
- 4,5,6,7-tetraiodoisobenzofuran-1,3-quinone
- 4,5,6,7-tetraiodo-2-benzofuran-1,3-dione
- 4,5,6,7-tetraiodoisobenzofuran-1,3-dione