ChemicalBook > CAS DataBase List > Sodium bisulfate
Sodium bisulfate
Sodium bisulfate
- CAS No.7681-38-1
- Chemical Name:Sodium bisulfate
- CBNumber:CB5854277
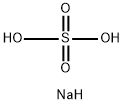
- Molecular Formula:NaHSO4
- Formula Weight:120.06
- MOL File:7681-38-1.mol
Sodium bisulfate Property
- Melting point 315 °C
- Density 2.1
- form Granules
- color White to light yellow
- Water Solubility soluble in 2 parts H2O, 1 part boiling H2O; decomposes in alcohol to sodium sulfate and H2SO4 [MER06]
- Sensitive Hygroscopic
- Merck 13,8658
- Stability Stable. Incompatible with strong bases, strong oxidizing agents, sodium carbonate, sodium hypochlorite. May decompose upon exposure to moist air or water.
- InChI InChI=1S/Na.H2O4S.H/c;1-5(2,3)4;/h;(H2,1,2,3,4);
- InChIKey TTXJTWGBBUPRBF-UHFFFAOYSA-N
- SMILES S(O)(O)(=O)=O.[NaH]
- CAS DataBase Reference 7681-38-1(CAS DataBase Reference)
- Indirect Additives used in Food Contact Substances SODIUM BISULFATE
- FDA 21 CFR 175.105
- EWG's Food Scores 1
- NCI Dictionary of Cancer Terms GBS
- FDA UNII BU8V88OWIQ
- EPA Substance Registry System Sodium bisulfate (7681-38-1)
- UNSPSC Code 12352302
- NACRES NA.23
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H318
- Precautionary statements P280-P305+P351+P338
Sodium bisulfate Price
More Price(6)
- Brand: Sigma-Aldrich(India)
- Product number: 307823
- Product name : Sodium hydrogen sulfate
- Purity: technical grade
- Packaging: 500G
- Price: ₹5661.48
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 307823
- Product name : Sodium hydrogen sulfate
- Purity: technical grade
- Packaging: 2.5KG
- Price: ₹12557
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 307823
- Product name : Sodium hydrogen sulfate
- Purity: technical grade
- Packaging: 20KG
- Price: ₹64040.7
- Updated: 2022/06/14
- Buy: Buy
- Brand: ALFA India
- Product number: ALF-B25587-30
- Product name : Sodium hydrogen sulfate, anhydrous, 90+%, remainder mainly sodium sulfate
- Purity:
- Packaging: 250g
- Price: ₹1697
- Updated: 2022/05/26
- Buy: Buy
- Brand: ALFA India
- Product number: ALF-B25587-0I
- Product name : Sodium hydrogen sulfate, anhydrous, 90+%, remainder mainly sodium sulfate
- Purity:
- Packaging: 5000g
- Price: ₹18925
- Updated: 2022/05/26
- Buy: Buy
Sodium bisulfate Chemical Properties,Usage,Production
-
Description
Sodium bisulfate is a white crystalline solid dissolved in water. It is corrosive to metals and tissue.
Sodium bisulfate is hygroscopic in that it attracts water. Sodium bisulfate dissociates completely in water into sodium (Na+), hydrogen (H+) and sulfate (SO4-2). As a mineral acid, sodium bisulfate is not expected to contaminate ground water or soil or to accumulate in the food chain (EPA 1993).

Sodium bisulfate is the sodium salt of sulfuric acid. It can be used as an acidifying/buffering agent in pesticide formulations applied to growing crops, as cooling and retort water treatment agent to inhibit corrosion on exteriors of canned goods, and as a feed additive. Sodium bisulfate is used as a disinfectant in the manufacture of foods and pickling compounds. It is used in the dye industry and in the textile industry during bleaching step. Sodium bisulfate is also used as an acid bath in the jewelry for pickling, which removes the surface fire scale and oxides from the metal, leaving it a bright silver color. -
Uses
Sodium bisulfate, or dry acid, is an acid salt known as sodium hydrogen sulfate. It can be used in a lot of ways such as food additives and cleaning, but in swimming pools, it's often used to lower pH balance and total alkalinity when they get too high. Porous aluminium can be fabricated by anodizing the metal in sodium hydrogen sulphate.
Sodium bisulfate is used as a top dressing to poultry litter to control ammonia in poultry houses. It is widely used in the commercial poultry industry (Blake and Hess 2001). It is also used in the dairy industry to reduce bacterial counts in bedding and ammonia emissions, preventing environmental mastitis and calf respiratory stress (Sun, et al. 2008). -
Production Methods
Historically sodium bisulfate is a by-product from the manufacture of nitric acid from sodium nitrate and sulfuric acid. The by-product is referred to as niter cake. Today there are two methods for producing sodium bisulfate. One involves mixing sodium hydroxide with sulfuric acid which will react to form sodium bisulfate and water as shown in the equation below. This method, produced by JOST Chemical® (Jost Chemical 2014), results in a sodium bisulfate monohydrate which is used as a laboratory reagent.
NaOH + H2SO4 → NaHSO4 + H2O
The petitioner states that they use another sodium bisulfate production method that involves reacting sodium chloride (salt) and sulfuric acid at elevated temperatures to produce sodium bisulfate and hydrogen chloride gas as shown in the equation below.
NaCl + H2SO4 → NaHSO4 + HCl
According to the petitioner, the liquid sodium bisulfate is then sprayed and cooled so that it forms solid beads. The hydrogen chloride gas produced is dissolved in water to produce hydrochloric acid, which may be sold as a by-product. -
References
[1] https://www.fda.gov
[2] Keith A. Jones, Environmentally safe pesticide compositions, Patent US 5739172A, 1998
[3] I.I. Kassem, Y.M. Sanad, R. Stonerock and G. Rajashekara, An evaluation of the effect of sodium bisulfate as a feed additive on Salmonella enterica serotype Enteritidis in experimentally infected broilers, Poult Sci., 2012, vol. 91, 1032-1037
[3] Ruth Winter, A Consumer's Dictionary of Food Additives, 7th Edition, 2009
[4] Ari Ben-Menahem, Historical Encyclopedia of Natural and Mathematical Sciences, Volume 1, 2009
[5] Sara Schwalbenberg, The Creation of Jewelry, Olin College, 2005 - Chemical Properties Also known as sodium acid sulfate, niter cake, sodium hydrogen sulfate, NaHS04, is colorless crystals or white fused lumps,whose aqueous solution is strongly acid.It is soluble in water and noncombustible. Derived as a byproduct in the manufacture of hydrochloric acid and nitric acid, it is purified by recrystallization. Used as a flux for decomposing minerals,substitute for sulfuric acid in dyeing,disinfectant, in the manufacture of sodium hydrosulfide,sodium sulfate,and soda slum,for liberating CO2 in carbonic acid baths,in thermophores, for carbonizing wool, in the manufacture of magnesia cements,paper,soap,perfumes, foods, industrial cleaners, metal pickling compounds, and as a lab reagent.
- Physical properties Colorless crystals; triclinic structure; density 2.435g/cm3 at 13°C; melts above 315°C; decomposes on further heating; soluble in water, 28.6 g/100mL at 25°C; highly soluble in boiling water, 100g/100 mL at 100°C; aqueous solution strongly acidic, pH of 0.1 M solution 1.4; insoluble in liquid ammonia; decomposed by alcohol into sodium sulfate and sulfuric acid.
- Uses sodium bisulfate is an inorganic salt used as an anti-septic and a pH adjuster in cosmetic creams. Concentrated solutions can produce strong irritation.
- Uses Sodium Hydrogen Sulfate is used in production of Nickel Hydroxide Electrode material for Alkaline battery.
- Uses Flux for decomposing minerals; substitute for sulfuric acid in dyeing; disinfectant; manufacture of sodium hydrosulfide, sodium sulfate, and soda alum; liberating CO 2 in carbonic acid baths, in thermophores; carbonizing wool; manufacture of magnesia cements, paper, soap, perfumes, foods, industrial cleaners, metal pickling compounds; lab reagent.
- General Description Bisulfate, aqueous solution is a white crystalline solid dissolved in water. Sodium bisulfate is corrosive to metals and tissue.
- Air & Water Reactions Dissolves in water to give strongly acidic solutions.
- Reactivity Profile Acidic salts, such as various BISULFATES, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. The combination of calcium hypochlorite, sodium hydrogen sulfate, starch, and sodium carbonate, when compressed, caused the materials to incandescence, followed by explosion, [Ind. Eng. Chem., 1937, 15, 282].
- Hazard Strong irritant to tissue.
- Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
- Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
- Safety Profile A corrosive irritant to skin, eyes, and mucous membranes. Mutation data reported. Reacts with moisture to form sulfuric acid. Uxtures with calcium hypochlorite + starch + sodium carbonate explode when compressed. Violent reaction with acetic anhydride + ethanol may lead to ignition and a vapor explosion. Incompatible with calcium hypochlorite. When heated to decomposition it emits toxic fumes of SO, and NanO. See also SULFATES.
Sodium bisulfate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(280)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15531157085<br/>+86-15531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Henan Fengda Chemical Co., Ltd
- Tel:+86-371-86557731<br/>+86-13613820652
- Email:info@fdachem.com
- Country:China
- ProdList:20241
- Advantage:58
-
Supplier:
Yujiang Chemical (Shandong) Co.,Ltd.
- Tel: +8617736087130
- Email:catherine@yjchem.com.cn
- Country:China
- ProdList:994
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21630
- Advantage:55
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Cangzhou Wanyou New Material Technology Co.,Ltd
- Tel:18631714998
- Email:sales@czwytech.com
- Country:CHINA
- ProdList:904
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Shandong chuangyingchemical Co., Ltd.
- Tel:18853181302
- Email:sale@chuangyingchem.com
- Country:CHINA
- ProdList:5906
- Advantage:58
-
Supplier:
Shanghai Longyu Biotechnology Co., Ltd.
- Tel: +8619521488211
- Email:info@longyupharma.com
- Country:China
- ProdList:2555
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39894
- Advantage:58
Related articles
7681-38-1, Sodium bisulfateRelated Search:
- Sodium formate Ferrous sulfate monohydrate Ferric sulfate Potassium sulfate Hydrogen Sodium acetate Sodium benzoate Neomycin sulfate Sodium hydroxide Sodium sulfate Ammonium sulfate Sodium carbonate Colistin sulfate Sodium bicarbonate Magnesium sulfate heptahydrate Sodium gluconate Sodium chloride Potassium bisulfate Sodium Bisulphate
- Synthetic Reagents
- Sodium Salts
- Sodium
- Salts
- Metal and Ceramic Science
- Materials Science
- Inorganic Salts
- Chemical Synthesis
- Inorganics
- 助剂
- 无机化合物
- 无机化工原料-无机盐
- 钠类
- 无机化工
- 其他
- 水处理药剂
- 其他危险化学品
- 大化工
- 袋装
- 有机类
- 能源材料
- 大化工产品
- 一般危化品
- 化工
- 通用生化试剂-无机盐
- 通用试剂
- 无机产品
- 精细化工品
- 有机盐
- 添加剂
- 化工原料
- 无机化工原料
- 有机化工原料
- 无机化工原料
- 矿物质硫酸盐
- 无机盐
- 有机化工原料类
- 催化和无机化学
- 微生物
- 钠
- Synthetic Reagents
- Inorganic Salts
- NHSO4H2O
- NaHSO4
- 681-38-1
- 硫酸氢钠(工业级)
- 焦性硫酸钠
- 硫酸氢钠, 无水, 90+%, 其余成份主要是硫酸钠
- 无水硫酸氢钠
- 硫酸氢钠,无水
- 硫酸氢钠,熔融
- 硫酸氢钠2
- 硫酸氢钠原料
- 硫酸氢钠
- 硫酸氢钠,酸式硫酸钠
- 焦性硫酸鈉
- 硫酸氫鈉
- 酸式硫酸钠