ChemicalBook > CAS DataBase List > Homoarginine
Homoarginine
Homoarginine
- CAS No.156-86-5
- Chemical Name:Homoarginine
- CBNumber:CB5298985
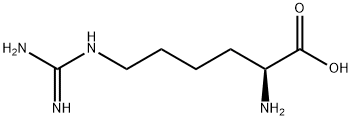
- Molecular Formula:C7H16N4O2
- Formula Weight:188.23
- MOL File:156-86-5.mol
Homoarginine Property
- Melting point 207-209 °C
- Boiling point 376.3±52.0 °C(Predicted)
- Density 1.39±0.1 g/cm3(Predicted)
- storage temp. Keep in dark place,Inert atmosphere,2-8°C
- solubility Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc.
- form Powder
- pka 2.52±0.24(Predicted)
- color White to off-white
- CAS DataBase Reference 156-86-5(CAS DataBase Reference)
- FDA UNII JF751CK38I
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H312-H332
- Precautionary statements P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P330-P363-P501
Homoarginine Chemical Properties,Usage,Production
-
Description
Homoarginine and 1-hydroxyhomoarginine were originally detected in significant concentration in the seeds of many Lathyrus species (Bell, 1962a,b; 1963). Homoarginine was isolated from the seeds of L. cicera (Bell, 1962b) and L. sativus (Rao et al., 1963). The hydroxy compound was obtained from the seeds of L. tingitanus (Bell, 1964). The comparative distribution of guanidino compounds in the seeds of two genus of the Papilionoideae, Lathyrus and Vicia, exhibits interesting features (Bell and Tirimanna, 1964). For example, the genus Lathyrus stores predominantly the C7 amino acids homoarginine, y-hydroxyhomoarginine and the related compound lathyrine, while the genus Vicia stores relatively high concentrations of canavanine and of the C6 amino acids arginine and y-hydroxyarginine. Apart from plants, homoarginine was found in small amounts in human body fluids ,. urine (Armstrong, 1967), serum (Matsumoto et al., 1976a) and cerebrospinal fluid (Mori et al., 1981b) and in mammalian brain (Mori et al., 1978, 1979 ). Increased urinary excretion was reported in hyperlysinemia (Armstrong and Robinow, 1967) and citrullinemia (Scott-Emuakpor et al., 19(2).
Homoarginine may be formed in mammals from lysine, by a homologous urea cycle in which arginine is replaced by lysine (Ryan and Wells, 1964; Scott-Emuakpor et al., 19(2). The excretion of homocitrulline and homoarginine in urine following administration of lysine to children supports this theory (Buergi et al., 1966). The biosynthesis of homoarginine has not been examined in plants, but y-hydroxyhomoarginine is synthesized from homoarginine in Lathyrus seeds (Bell, 1964). Subsequently both compounds have been identified as the precursors of the cyclic guanidino derivative, lathyrine, also present in Lathyrus seeds (Bell and Przybylska, 1965). Homoarginine and y-hydroxyhomoarginine probably serve as nitrogen reserves in Lathyrus seeds.
Inhibition of growth by homoarginine has been reported In various microorganisms (Walker, 1955; Rao et al., 1963).
gama-Hydroxyhomoarginine lactone isolated from the seeds of L. tingitanus (Bell, 1964) probably results from the cyclization of the open chain compound under acidic conditions. - Chemical Properties White to off-white powder
- Uses Homoarginine is an inhibitor of human arginase activity.
-
Uses
Homoarginine as marker for exogenous protein
Absorption and secretion of free homoarginine in the intestine: when 9 mmol homoarginine were infused into the proximal part of the jejunum, there was a 99.9% disappearance of the free amino acid homoarginine up to the ileum. In addition, following the infusion of 9 mmol homoarginine into the vena jugularis less than 0.2% of the infused homoarginine appeared in the intestine.
We concluded from these data that with the homoarginine method endogenous protein in the chyme of the intestine can be distinguished from the food protein as homoarginine is not incorporated into endogenous secreta and free homo arginine is completely absorbed. - Definition ChEBI: An L-lysine derivative that is the L-enantiomer of homoarginine.
- IC 50 Alkaline phosphatase; Human Endogenous Metabolite
Homoarginine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(197)Suppliers
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19808
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
156-86-5, HomoarginineRelated Search:
- ARGINASE L(+)-Arginine L-HOMOCITRULLINE (S)-4-ACETOXY-2-AMINO-BUTYRIC ACID H-HOMOPRO-OME HCL H-HomoPhe-OtBu.HCl (αS)-α-AMino-benzenebutanoic Acid Methyl Ester Hydrochloride L-BETA-HOMOLEUCINE HYDROCHLORIDE H-HomoArg(Boc)2-OH arginine aspirin L(+)-Homoarginine hydrochloride BOC-N-OMEGA-(NITRO)-L-HOMOARGININE,BOC-HOMOARGININE(NO2)-OH,Boc-N-Nitro-L-HomoArginine D-Homoarginine hydrochloride BOC-HOMOARGININE HCL,BOC-L-HOMOARGININE BOC-L-BETA-HOMOARGININE(TOS) FMOC-HOMOARGININE(PMC)-OH,FMOC-N-OMEGA-(2,2,5,7,8-PENTAMETHYLCHROMAN-6-SULFONYL)-L-HOMOARGININE 6-GUANIDINOHEXANOIC ACID FMOC-L-HOMOARGININE, HYDROCHLORIDE SALT
- Unusual Amino Acids
- Chiral Compound
- Amino Acids
- 抑制剂
- 药靶配体
- 中药对照品
- 对照品
- 中间体
- 非天然氨基酸
- 保护氨基酸
- 氨基酸
- 氨基酸衍生物
- 医药中间体
- 其他生化试剂
- 化学生物学
- 高精氨酸 L-高精氨酸 CP级 156-86-5
- 高精氨酸对照品
- 高精氨酸,95%
- H-高精氨酸
- L-高精氨酸 5G
- 類鳥氨酸
- 高精氨酸
- 156-86-5
- Valine Impurity 28
- )-olean-12-ene-3,28-diol
- (3β
- Homoarginine reference substance
- L-Homo-Arginine-OH
- Homoarginine,97%
- Homoarginine USP/EP/BP
- 1-(1,1,2,2-tetrafluoroethoxy)-4-[2-[4-(1,1,2,2-tetrafluoroethoxy)phenyl]propan-2-yl]benzene
- L-HomoArg-OH
- L-Homoarginine≥ 99% (Assay)
- L-a-AMino-e-guanidinohexanoic acid
- H-HoMoarg-OH(H-HoMoarginine)
- C01924
- N6-Amidino-L-lysine
- L-α-Amino-ε-guanidinohexanoic acid
- (S)-2-AMINO-6-GUANIDINOHEXANOIC ACID
- L-LYSINE, N6-(AMINOIMINOMETHYL)-
- H-HOMOARGININE
- Homo-L-arginine
- (2S)-2-amino-6-(diaminomethylideneamino)hexanoic acid
- HOMOARGININE
- H-HAR-OH
- H-HOARG-OH
- H-HOMOARG-OH
- L-ALPHA-AMINO-EPSILON-GUANIDINOHEXANOIC ACID
- L-HOMOARGININE
- n(sup6)-(aminoiminomethyl)-l-lysin
- l-n(sup6)-amidinolysine
- l-lysin