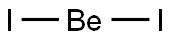ChemicalBook > CAS DataBase List > BERYLLIUM IODIDE
BERYLLIUM IODIDE
BERYLLIUM IODIDE
- CAS No.7787-53-3
- Chemical Name:BERYLLIUM IODIDE
- CBNumber:CB5246315
- Molecular Formula:BeI2
- Formula Weight:262.82
- MOL File:7787-53-3.mol
BERYLLIUM IODIDE Property
- Melting point 480°
- Boiling point bp 488°
- Density 4.320
- solubility reacts with H2O; soluble in ethanol
- form hygroscopic needles
- color colorless needle-like crystals
- Water Solubility reacts violently with H2O giving off HI [MER06]
- FDA UNII 9E9VD36EWN
- EPA Substance Registry System Beryllium iodide (BeI2) (7787-53-3)
Safety
- HS Code :2827600090
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
BERYLLIUM IODIDE Chemical Properties,Usage,Production
- Chemical Properties -60 mesh, 99.5% pure and needles; very hygroscopic; enthalpy of vaporization 70.5 kJ/mol; enthalpy of fusion 21.00 kJ/mol; obtained by reaction of Be and I2 at 500°C–700°C [CER91] [KIR78] [MER06] [CRC10]
-
Preparation
Beryllium iodide has the formula BeI2. It is very
hygroscopic and reacts violently with water, forming
hydroiodic acid. Beryllium iodide can be prepared by
reacting Be metal with elemental iodine at temperatures
of 500°C–700°C:
Be+I2→BeI2
Beryllium iodide is also formed when beryllium carbide reacts with hydrogen iodide in the gas phase:
Be2C+4HI→2BeI2+CH4
Beryllium Iodide, BeI2, was first prepared by W?hler (1828) and Debray (1855) who prepared the iodide by the action of iodine upon the metal. Lebeau (1898), was the first to prepare large quantities (for the time) by the action of gaseous hydroiodic acid, or a mixture of hydrogen and iodine vapor, on the carbide at about 700°C.
Beryllium iodide, as obtained in the sublimed state, consists of colorless crystals, which are quickly decomposed in moist air. Its specific gravity at 15°C is close to 4.20 g/cm3. It begins to sublime below its melting point which is 510°C. The melted iodide boils between 585 and 595°C. It is insoluble in benzene, toluene, spirits of turpentine, and but slightly soluble in carbon disulphide. The slightest trace of water attacks it immediately, but if it is first fused, its sensitivity to water is much less. It can be distilled without alteration in dry hydrogen, nitrogen or carbon dioxide. At this point in time, the structure BeI2 has not been published.
BERYLLIUM IODIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(10)Suppliers
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8772
- Advantage:58
-
Supplier:
Hu Bei Jiutian Bio-medical Technology CO.,Ltd
- Tel:027-88013699<br/>17354350817
- Email:Ryan@jiutian-bio.com
- Country:China
- ProdList:6000
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: Shaanxi DIDU pharmaceutical and Chemical Co., Ltd
- Tel:029-029-81148696<br/>18161915376
- Email:1040@dideu.com
- Country:China
- ProdList:9799
- Advantage:58
7787-53-3, BERYLLIUM IODIDERelated Search:
- 铍碘化物
- 碘化铍
- 7787-53-3
- Reaxys ID: 15952956
- BERYLLIUM IODIDE
- Einecs 232-119-0
- Berylliumiodide99.5%
- beryllium diiodide
- Beryllium iodide (BeI2)
- BeI2