ChemicalBook > CAS DataBase List > Medetomidine hydrochloride
Medetomidine hydrochloride
Medetomidine hydrochloride
- CAS No.86347-15-1
- Chemical Name:Medetomidine hydrochloride
- CBNumber:CB52130917
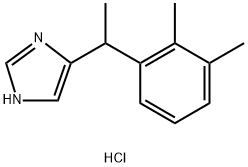
- Molecular Formula:C13H17ClN2
- Formula Weight:236.74
- MOL File:86347-15-1.mol
Medetomidine hydrochloride Property
- Melting point 164-166°C
- storage temp. Inert atmosphere,Store in freezer, under -20°C
- solubility Acetonitrile (Slightly), Methanol (Slightly), Water (Slightly)
- form Solid
- color White to Off-White
- biological source synthetic
- InChI InChI=1S/C13H16N2.ClH/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13;/h4-8,11H,1-3H3,(H,14,15);1H
- InChIKey VPNGEIHDPSLNMU-UHFFFAOYSA-N
- SMILES C(C1N=CNC=1)(C1C=CC=C(C)C=1C)C.Cl
- FDA UNII BH210P244U
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H317-H319
- Precautionary statements P280-P305+P351+P338
Medetomidine hydrochloride Chemical Properties,Usage,Production
- Description Medetomidine hydrochloride is a potent, highly selective α2-adrenoceptor agonist (Ki values are 1.08 and 1750 nM for α2- and α1-adrenoceptors respectively). It is an imidazole compound. Medetomidine hydrochloride shows greater selectivity over α1-adrenoceptors than clonidine and UK 14,304 (1620-, 220- and 300-fold respectively). In addition, it influences the adrenergic system by mimicking the action of adrenergic neurotransmitters.
- Chemical Properties White Solid
- Uses An α2-Adrenergic agonist. Sedative; analgesic.
- Uses Medetomidine is a selective α2-adrenoceptor agonist, with Ki of 1.08 nM, exhibts 1620-fold selectivity over α1-adrenoceptor
- Definition ChEBI: Medetomidine hydrochloride is a hydrochloride.
- Indications Medetomidine hydrochloride is an FDA-approved alpha2-adrenoceptor (α2-AR) agonist with analgesic, sedative and anxiolytic properties for use as a veterinary sedative. It has been approved for use in animals such as cats, sheep, and dogs (12 weeks of age and older.) In 1999, the FDA approved Medetomidine for human use in the sedation of intubated and mechanically ventilated intensive care unit (ICU) patients, including use as a pain reliever and for use in non-intubated patients prior to and during surgery and other procedures. In addition to its pharmaceutical uses, it is used as an antifouling agent in marine coatings.[1]
- Biological Activity medetomidine is a selective α2-adrenoceptor agonist, with ki of 1.08 nm, exhibts 1620-fold selectivity over α1-adrenoceptor.
- Pharmacokinetics After intramuscular injection, medetomidine is rapidly and almost completely absorbed at the site of injection and its pharmacokinetics are very similar to that observed after intravenous injection. Maximum plasma concentrations are reached within 15 to 20 minutes. Estimated plasma half-life is 1.2 hours for dogs and 1.5 hours for cats. Medetomidine is mainly oxidised in the liver, while a small amount is methylated in the kidney. Metabolites are primarily excreted in urine.
- Side effects Medetomidine hydrochloride is an alpha-2 adrenoreceptor agonist. Symptoms after absorption may involve clinical effects, including dose dependent sedation, respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycaemia. Ventricular arrhythmias have also been reported. Respiratory and haemodynamic symptoms should be treated symptomatically.
-
Synthesis
Medetomidine hydrochloride is synthesized using 2,3-Dimethylbromobenzene as the starting material through a series of Grignard reactions. This process yields tertiary alcohol 7, which is subsequently subjected to dehydration and hydrogenation. The overall yield achieved was 17 percent.[1] The reaction scheme is illustrated as follows:
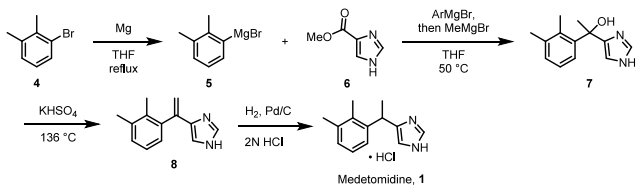
Additional synthetic processes encompass: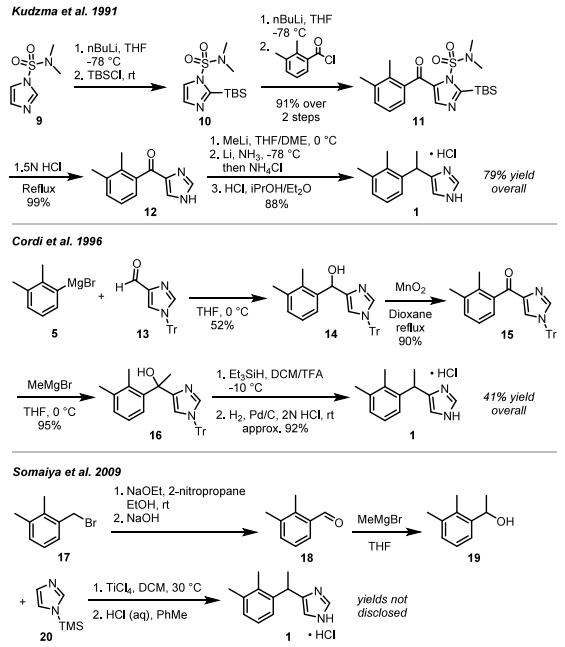
- storage Desiccate at RT
- References [1] PEDRO DE ANDRADE HORN. Classics in Chemical Neuroscience: Medetomidine.[J]. ACS Chemical Neuroscience, 2024: 3874-3883. DOI:10.1021/acschemneuro.4c00583.
Medetomidine hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(246)Suppliers
-
Supplier:
Guangdong Junhua Chemical Co., Ltd
- Tel:+86-075785239927<br/>+86-13326778444
- Email:aron@hjococ.com
- Country:China
- ProdList:42
- Advantage:58
-
Supplier:
Baoji Guokang Healthchem co.,ltd
- Tel:+8615604608665<br/>15604608665
- Email:dominicguo@gk-bio.com
- Country:CHINA
- ProdList:9414
- Advantage:58
-
Supplier:
Wuhan Golt Biotech Co., Ltd.
- Tel: +8615389281203
- Email:maria@goltbiotech.com
- Country:China
- ProdList:970
- Advantage:58
-
Supplier:
Guangzhou Tengyue Chemical Co., Ltd.
- Tel:+86-86-18148706580<br/>+8618826483838
- Email:evan@tyvovo.com
- Country:China
- ProdList:146
- Advantage:58
-
Supplier:
Guangzhou Tosun Pharmaceutical Ltd
- Tel:+86-020-61855200-902<br/>+8618124244216
- Email:info@upharm.cn
- Country:China
- ProdList:897
- Advantage:58
-
Supplier:
Shandong Xuhuang New material CoLTD
- Tel:+86-15095020839<br/>+86-17660422208
- Email:
- Country:China
- ProdList:726
- Advantage:58
-
Supplier:
shandong perfect biotechnology co.ltd
- Tel:+86-53169958659<br/>+86-13153181156
- Email:sales@sdperfect.com
- Country:China
- ProdList:294
- Advantage:58
-
Supplier:
Shandong Huisheng Import & Export Co., Ltd.
- Tel:+86-13176845580<br/>+86-13176845580
- Email:da@zhongda-biotech.com
- Country:China
- ProdList:232
- Advantage:58
-
Supplier:
Hebei Xinsheng New Material Technology Co., LTD.
- Tel: +86-16632316109
- Email:xinshengkeji@xsmaterial.com
- Country:China
- ProdList:1085
- Advantage:58
-
Supplier:
Shandong Hanjiang Chemical Co., Ltd
- Tel:+86-0533-2066820<br/>+8618369939125
- Email:hanson@sdhanjiang.com
- Country:China
- ProdList:339
- Advantage:58
86347-15-1, Medetomidine hydrochlorideRelated Search:
- 1-Vinylimidazole Ethyl acrylate N,N-Dimethylformamide Topotecan hydrochloride Cyproheptadine hydrochloride sesquihydrate Ethanol Chlorodimethylphenylsilane Imidazole Ethyl acetate Dexmedetomidine hydrochloride Triprolidine hydrochloride Medetomidine Ethylparaben Diethyl ether MEPERIDINE HYDROCHLORIDE Medetomidine HCl
- Inhibitors
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Aromatics
- 抑制剂
- 科研原料
- 药靶配体
- 化工原料
- 化学试剂
- 精细化工原料
- 粉末类
- 药物杂质及中间体
- 医药原料和中间体
- 原料药
- 化工
- 盐酸美托咪啶
- 医药原料
- 保健与医疗
- 止痛类
- 神经信号
- 医药原料药
- 小分子抑制剂,天然产物
- 化合物MEDETOMIDINE HCL,10 MM DMSO 溶液
- MEDETOMIDINE HYDROCHLORIDE,ALPHA2 ADRENOCEPTOR激动剂
- MEDETOMIDINE HYDROCHLORIDE,ALPHA2 ADRENOCEPTOR激动剂(AQUEOUS SOLUTION)
- 化合物MEDETOMIDINE HCL
- 盐酸美托咪啶杂质
- 盐酸美托咪啶/盐酸美托咪定
- 4-(1-(2,3-二甲基苯基)乙基)-1H咪唑盐酸盐
- 2,3-二甲基苯基
- 3-二甲基苯基)乙基]-1H-咪唑盐酸盐
- 盐酸美托嘧啶
- 5-[1-(2,3-二甲基苯基)乙基]-1H-咪唑盐酸盐
- 美托咪定盐酸盐
- 盐酸咪托咪定
- 盐酸美托咪定
- (R)-4-[1-(2,3-二甲基苯基)乙基]-1H-咪唑盐酸盐
- 盐酸美托咪啶
- 86347-15-1
- Medetomidine hydrochloride, 10 mM in DMSO
- Medetomidine hydrochloride, alpha2 adrenoceptor agonist
- Medetomidine hydrochloride, alpha2 adrenoceptor agonist (aqueous solution)
- Ethyl Acetate Impurity 85
- Medetomidine HCl Powder
- Medetomidine hydrochloride,anxiolysis,inhibit,sedation,Medetomidine,α2-adrenoceptor,MPV-785,peripheral nervous system,analgesia,MPV 785,central nervous system,Inhibitor,muscle relaxation,Beta Receptor,Adrenergic Receptor
- MEDETOMIDINE HYDROCHLORIDE
- Medetomidine HCl (MPV-785)
- medeomidine hcl
- Metoclopramide (metoclopramide)
- Medetomidine Hydrochloride Impurities
- (R)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride USP/EP/BP
- Medetomidine Hcl Medetomidine Hydrochloride
- 2-amino-5-(2-ethoxyanilino)-4-hydroxy-1H-pyrimidin-6-one
- SIMS1031 Medetomidine HCl ---86347-15-1 25g
- Medetomidine (hydrochloride) cas86347-15-1
- 3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride
- (R)-4-[1-(2