ChemicalBook > CAS DataBase List > L-Homocystine
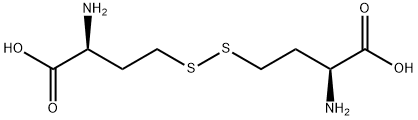
L-Homocystine
L-Homocystine
- CAS No.626-72-2
- Chemical Name:L-Homocystine
- CBNumber:CB5155903
- Molecular Formula:C8H16N2O4S2
- Formula Weight:268.35
- MOL File:626-72-2.mol
L-Homocystine Property
- Melting point 281-284 °C (dec.)(lit.)
- alpha 77 º (c=1% in 1N HCl)
- Boiling point 507.6±50.0 °C(Predicted)
- Density 1.443±0.06 g/cm3(Predicted)
- storage temp. Keep in dark place,Inert atmosphere,Room temperature
- solubility H2O : 5 mg/mL (18.63 mM; ultrasonic and adjust pH to 3 with H2O)DMSO : 1 mg/mL (3.73 mM; ultrasonic and adjust pH to 5 with HCl)
- form Powder
- pka 1.90±0.10(Predicted)
- color Off-white to light yellow
- BRN 1728583
- InChIKey ZTVZLYBCZNMWCF-WDSKDSINSA-N
- CAS DataBase Reference 626-72-2(CAS DataBase Reference)
- FDA UNII 804AS222UE
- EPA Substance Registry System L-Homocystine (626-72-2)
- UNSPSC Code 12352209
- NACRES NA.26
Safety
- Safety Statements :22-24/25
- WGK Germany :3
- HS Code :29309090
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
L-Homocystine Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: H6010
- Product name : L-Homocystine
- Purity: ≥98% (HPLC)
- Packaging: 100MG
- Price: ₹8421.85
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: H6010
- Product name : L-Homocystine
- Purity: ≥98% (HPLC)
- Packaging: 500MG
- Price: ₹18803.03
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: H6010
- Product name : L-Homocystine
- Purity: ≥98% (HPLC)
- Packaging: 1G
- Price: ₹32345.1
- Updated: 2022/06/14
- Buy: Buy
L-Homocystine Chemical Properties,Usage,Production
- Chemical Properties L-homocysteine is an aliphatic amino acid with the molecular formula (C8H16N2O4S2). It is a white powder with an acidic taste and is easily soluble in water. It has limited solubility in ethanol. L-homocysteine has a sulfhydryl group on its side chain, which allows it to form disulfide bonds with other L-homocysteine residues in proteins. This feature contributes to the stable three-dimensional structure of proteins. As a result, L-homocysteine finds extensive applications in industries such as pharmaceuticals, food, feed, and cosmetics.
- Uses Increased levels lead to hyperhomocysteinemia; a cardiovascular risk factor in prediction of coronary heart disease as well as being associated with congenital birth defects, pregnancy complications and cancer.
- Definition ChEBI: L,L-homocystine is a homocystine in which both chiral centres have L configuration. It is functionally related to a L-homocysteine. It is a tautomer of a L,L-homocystine zwitterion.
- Biological Activity L-Homocystine is the oxidized member of the L-homocysteine:L-homocystine pair. Homocysteine/homocystine is derived from methionine. Homocysteine is a pro-thrombotic factor, vasodilation impairing agent, pro-inflammatory factor and endoplasmatic reticulum-stress inducer used to study cardiovascular disease mechanisms.
-
Synthesis
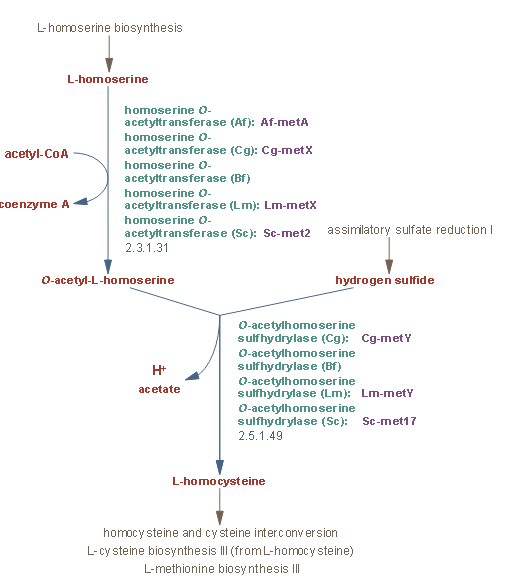
L-homoserine(626-72-2) is biosynthesized from L-aspartate, which is synthesized from oxaloacetate, a TCA cycle intermediate. Homoserine is activated through esterification to form the O-acetyl ester by the enzyme homoserine O-acetyltransferase. In certain organisms, including the yeast Saccharomyces cerevisiae and certain bacteria, hydrogen sulfide reacts with O-acetyl-L-homoserine, replacing the acetyl group and forming L-homocysteine.
- Purification Methods The acid (3g) is dissolved in freshly boiled H2O (30mL) under N2, cooled under N2 (all operations should be under N2), absolute EtOH (100mL) is added, the acid is filtered off, and a second crop is obtained by diluting the filtrate to 500mL with absolute EtOH, kept overnight in a refrigerator, filtered, washed with EtOH and dried in a vacuum. The D(R,R)-form has similar properties but is –ve in M HCl and +ve in H2O. [du Vigneaud & Patterson J Biol Chem 109 101 1935, du Vigneaud & Brown Biochemical Preparations 5 93, 95 1975, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2667-2670 1961, Beilstein 4 III 1643, 4 IV 3199, Koegel et al. J Am Chem Soc 77 5708 1955.]
L-Homocystine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(250)Suppliers
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Accela ChemBio Inc.
- Tel:+1-858-6993322
- Email:info@accelachem.com
- Country:United States
- ProdList:19825
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-87569262<br/>+86-15003564040
- Email:1087@dideu.com
- Country:China
- ProdList:3254
- Advantage:58
-
Supplier:
Antai Fine Chemical Technology Co.,Limited
- Tel: 18503026267
- Email:info@antaichem.com
- Country:CHINA
- ProdList:9636
- Advantage:58
626-72-2, L-HomocystineRelated Search:
- 4-Aminobutyric acid D-2-Aminobutyric acid N-Acetyl-L-cysteine DL-Homocysteine L-Cystine DL-2-AMINOBUTYRIC ACID L-Cysteine Selenium sulfide DL-3-Aminobutyric acid Cyproterone acetate Indole-3-butyric acid DL-2-Aminobutyric acid L(+)-2-Aminobutyric acid 3-CARBOXYPROPYL DISULFIDE Butyl disulfide Methyl propyl disulfide r-Amino Butryric Acid ETHYL PROPYL DISULFIDE
- Amino Acids
- Sulfur & Selenium Compounds
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Amines
- 杂质对照品
- 香精香料
- 生化试剂
- 精细化工原料
- 食品添加剂
- 化学原料
- 抑制剂
- 中药对照品
- 生化试剂-氨基酸类
- 化学试剂
- 对照品
- 非天然氨基酸
- 保护氨基酸
- 氨基酸
- 化学生物学
- 非天然氨基酸衍生物
- 氨基酸类
- Homo-Amino Acids
- Peptide Synthesis
- Specialty Synthesis
- Unnatural Amino Acid Derivatives
- C8H16N2O4S2
- L-高胱氨酸L-HOMOCYSTINE
- <SC>L </SC>-高胱氨酸
- L-类胱氨酸/L-同型胱氨酸/同型半胱氨酸/高胱氨酸
- L-类胱氨酸、L-同型胱氨酸、L-4,4'-二硫双(2-氨基丁酸)
- 同型半胱氨酸,高胱氨酸
- L-同型半胱氨酸
- 4'-二硫双(2-氨基丁酸) H-HOCYS-OH
- L-高胱氨酸,98%
- L-4,4'-二硫双(2-氨基丁酸) H-HOCYS-OH
- L-4,4'-二硫双(2-氨基丁酸)
- L-高胱氨酸
- L-同型胱氨酸
- L-类胱氨酸
- 626-72-2
- <sc>L</sc>-Homocystine
- Vincamine Impurity 1( Vincamine EP Impurity A )
- L Homocystine,Inhibitor,Endogenous Metabolite,LHomocystine,inhibit,L-Homocystine
- (2S,2'S)-4,4'-Disulfanediylbis(2-aMinobutanoic acid)
- (2S)-2-amino-4-{[(3S)-3-amino-3-carboxypropyl]disulfanyl}butanoic acid
- Butanoic acid, 4,4'-dithiobis[2-amino-, (2S,2'S)-
- L-Homocystine USP/EP/BP
- H-L-HCystine
- L-Homocystine
- (H-Hcys-OH) 2
- H-HoCys-OH
- 2-amino-4-(3-amino-3-carboxy-propyl)disulfanyl-butanoic acid
- [S-(R*,R*)]-4,4'-dithiobis[2-aminobutyric] acid
- L-homocystine cell culture tested
- (H-Hcy-OH)?
- 4,4’-dithiobis[2-amino-,[s-(theta,theta)]-butanoicaci
- L-4,4'-DITHIO-BIS(2-AMINOBUTANOIC ACID)