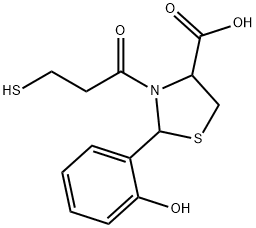
rentiapril
- CAS No.72679-47-1
- Chemical Name:rentiapril
- CBNumber:CB51321949
- Molecular Formula:C13H15NO4S2
- Formula Weight:313.39
- MOL File:72679-47-1.mol
- Boiling point 553.7±50.0 °C(Predicted)
- Density 1.451±0.06 g/cm3(Predicted)
- storage temp. Store at -20°C
- solubility Soluble in DMSO
- form Solid
- pka 3.02±0.40(Predicted)
- color White to off-white
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
rentiapril Chemical Properties,Usage,Production
- Uses Rentiapril racemate (SA-446 racemate) is the racemate of Rentiapril. Rentiapril is an angiotensin converting enzyme (ACE) inhibitor.
-
in vivo
A three-months toxicity study of an angiotensin converting enzyme (ACE) inhibitor, Rentiapril (CAS 80830-42-8), is performed in Sprague-Dawley rats by oral administration. The dose levels of 0, 30, 125, 500 and 1000 mg/kg are tested in both sexes, in which each experimental group comprised 10 rats. Another ACE inhibitor, captopril, is used as a reference compound. Rentiapril at the highest dose of 1000 mg/kg causes low food consumption and death of some animals with signs of bloody feces and anemia. In males and females receiving 500 and 1000 mg/kg, there are low body weight gain, increases in water intake, urine volume and serum BUN level, and decreases in levels of various erythrocytic parameters. Kidney weight is increased dose-dependently in both sexes. Histopathologically, renal changes in the 500 and 1000 mg/kg groups consist of proximal tubular degeneration, juxtaglomerular cell hyperplasia and interstitial cell infiltration. Similar, but mild, changes in proximal tubules are present in the female 125 mg/kg group. Dead animals from the highest dose groups further show gastrointestinal hemorrhagic erosion and/or ulcer, decrease bone marrow erythropoiesis and hepatocytic vacuolar degeneration. There is no pathological alteration in rats from other Rentiapril-treated groups, as well as in controls. These results indicate that the no-effect dose of Rentiapril in rats by three months oral administration is 30 mg/kg in female and 125 mg/kg in male[1].
- References [1] Takase K, et al. Toxicity study of the angiotensin converting enzyme inhibitor rentiapril in rats. Arzneimittelforschung. 1995 Jan;45(1):15-8. PMID:7893262
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32251
- Advantage:58
- Supplier: LGM Pharma
- Tel:1-(800)-881-8210
- Email:inquiries@lgmpharma.com
- Country:United States
- ProdList:2123
- Advantage:70
- Supplier: MedChemexpress LLC
- Tel:021-58955995
- Email:sales@medchemexpress.cn
- Country:United States
- ProdList:4861
- Advantage:58
- Supplier: Fan De(Beijing) Biotechnology Co., Ltd.
- Tel: 15911056312
- Email:liming@bio-fount.com
- Country:China
- ProdList:9729
- Advantage:58
- Supplier: Beijing Jin Ming Biotechnology Co., Ltd.
- Tel:010-60605840<br/>15801484223;
- Email:psaitong@jm-bio.com
- Country:China
- ProdList:29774
- Advantage:58
- Supplier: TargetMol Chemicals Inc.
- Tel: 4008200310
- Email:marketing@tsbiochem.com
- Country:China
- ProdList:24762
- Advantage:58
- Supplier: Shanghai Yifei Biotechnology Co. , Ltd.
- Tel:021-65675885<br/>18964387627
- Email:customer_service@efebio.com
- Country:China
- ProdList:11973
- Advantage:58
- Supplier: RD International Technology Co., Limited
- Tel: 18024082417
- Email:market@ubiochem.com
- Country:China
- ProdList:9835
- Advantage:58
- Supplier: Sangon Biotech (Shanghai) Co.,Ltd.
- Tel:400-821-026
- Email:Sales@sangon.com
- Country:China
- ProdList:48813
- Advantage:58
- 抑制剂
- 化合物 T16731
- 伦噻普利外消旋体
- 伦唑普利外消旋体
- 72679-47-1
- SA-446 racemate
- 2-(2-hydroxyphenyl)-3-(3-sulfanylpropanoyl)-1,3-thiazolidine-4-carboxylic acid
- Rentiapril (racemate)
- 4-Thiazolidinecarboxylic acid, 2-(2-hydroxyphenyl)-3-(3-mercapto-1-oxopropyl)-
- 2-(2'-Hydroxyphenyl)-3-(3-mercaptopropanoyl)-4-thiazolidine carboxylic acid