ChemicalBook > CAS DataBase List > 3-(TRIFLUOROACETYL)INDOLE
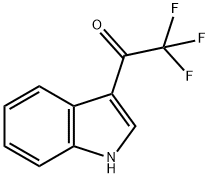
3-(TRIFLUOROACETYL)INDOLE
3-(TRIFLUOROACETYL)INDOLE
- CAS No.14618-45-2
- Chemical Name:3-(TRIFLUOROACETYL)INDOLE
- CBNumber:CB5129548
- Molecular Formula:C10H6F3NO
- Formula Weight:213.16
- MOL File:14618-45-2.mol
3-(TRIFLUOROACETYL)INDOLE Property
- Melting point 211-214 °C(lit.)
- Boiling point 308.6±37.0 °C(Predicted)
- Density 1.423±0.06 g/cm3(Predicted)
- storage temp. Sealed in dry,Room Temperature
- pka 14.31±0.30(Predicted)
- form Solid
- color White to Almost white
- CAS DataBase Reference 14618-45-2(CAS DataBase Reference)
- UNSPSC Code 12352100
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P280a-P304+P340-P305+P351+P338-P405-P501a
3-(TRIFLUOROACETYL)INDOLE Price
More Price(2)
- Brand: TCI Chemicals (India)
- Product number: T1809
- Product name : 3-(Trifluoroacetyl)indole
- Purity: min. 98.0 %
- Packaging: 1G
- Price: ₹5100
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T1809
- Product name : 3-(Trifluoroacetyl)indole
- Purity: min. 98.0 %
- Packaging: 5G
- Price: ₹14700
- Updated: 2022/05/26
- Buy: Buy
3-(TRIFLUOROACETYL)INDOLE Chemical Properties,Usage,Production
- Uses Reactant for enantioselective synthesis of indoles via palladium-catalyzed kinetic asymmetrical alkylation. Reactant for preparation of N-pyridinyl indolecarboxamides via amidation of aminopyridine derivatives. with indolecarboxylic acid 2 Reactant for preparation of mast cell tryptase inhibitors, starting from indoles; using amidation as the key step 3 Reactant for formation of Michael adducts via Baylis-Hillman reaction 4 Reactant for preparation of indolecarboxamides via haloform cleavage of (trifluoroacetyl)indole with lithium dialkylamides.
-
Uses
- Reactant for enantioselective synthesis of indoles via palladium-catalyzed kinetic asymmetrical alkylation
- Reactant for preparation of N-pyridinyl indolecarboxamides via amidation of aminopyridine derivatives. with indolecarboxylic acid
- Reactant for preparation of mast cell tryptase inhibitors, starting from indoles; using amidation as the key step
- Reactant for formation of Michael adducts via Baylis-Hillman reaction
- Reactant for preparation of indolecarboxamides via haloform cleavage of (trifluoroacetyl)indole with lithium dialkylamides
- General Description 3-(Trifluoroacetyl)indole is a heterocyclic trifluoromethyl ketone. One of the methods reported for its synthesis is by the reaction of indole with trifluoroacetic anhydride.
3-(TRIFLUOROACETYL)INDOLE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(91)Suppliers
-
Supplier:
Shenzhen Nexconn Pharmatechs Ltd
- Tel:+86-755-89396905<br/>+86-15013857715
- Email:admin@nexconn.com
- Country:China
- ProdList:10406
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
14618-45-2, 3-(TRIFLUOROACETYL)INDOLERelated Search:
- 1-Methyl-3-(trifluoroacetyl)-1H-indole 1-(1-ETHYL-1H-INDOL-3-YL)-2,2,2-TRIFLUORO-1-ETHANONE 3-(TRIFLUOROACETYL)INDOLE 2,2,2-TRIFLUORO-1-[1-(METHYLSULFONYL)-1H-INDOL-3-YL]-1-ETHANONE 2,2,2-trifluoro-1-(5-nitro-1H-indol-3-yl)ethanone 2,2,2-TRIFLUORO-1-(2-PHENYL-1H-INDOL-3-YL)-1-ETHANONE 1-(1-BENZYL-1H-INDOL-3-YL)-2,2,2-TRIFLUORO-1-ETHANONE
- Heterocyclic Building Blocks
- Building Blocks
- Simple Indoles
- Plant Growth Trgulators (Others)
- Plant Growth Regulators
- Indoles
- Biochemistry
- 高端化学
- 合成材料中间体
- 杂环化合物
- Heterocyclic Building Blocks
- Plant Growth Regulators
- Plant Growth Trgulators (Others)
- Simple Indoles
- Indoles
- Building Blocks
- 2,2,2-三氟-1-(1H-吲哚-3-基)-1-乙酮
- 3-(三氟乙酰)-吲哚
- 3-( 三氟乙酰基 ) 吲哚
- 14618-45-2
- Ethanone, 2,2,2-trifluoro-1-(1H-indol-3-yl)-
- 3-Indolyl trifluoromethyl ketone
- 3-(TRIFLUOROACETYL)INDOLE 99%
- 3-(TRIFLUOROACETYL)INDOLE
- 2,2,2-TRIFLUORO-1-(1H-INDOL-3-YL)-1-ETHANONE