ChemicalBook > CAS DataBase List > FUSARIC ACID
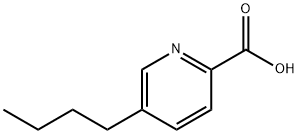
FUSARIC ACID
FUSARIC ACID
- CAS No.536-69-6
- Chemical Name:FUSARIC ACID
- CBNumber:CB4689029
- Molecular Formula:C10H13NO2
- Formula Weight:179.22
- MOL File:536-69-6.mol
FUSARIC ACID Property
- Melting point 96-100 °C
- Boiling point 311.75°C (rough estimate)
- Density 1.1248 (rough estimate)
- refractive index 1.5710 (estimate)
- storage temp. Inert atmosphere,Store in freezer, under -20°C
- solubility ethanol: 50 mg/mL, clear, faintly yellow
- pka 1.11±0.50(Predicted)
- form Crystalline Powder
- color Off-white to faint yellow
- Merck 14,4314
- BRN 125804
- LogP 1.960 (est)
- CAS DataBase Reference 536-69-6(CAS DataBase Reference)
- FDA UNII JWJ963070N
- EPA Substance Registry System 2-Pyridinecarboxylic acid, 5-butyl- (536-69-6)
- UNSPSC Code 12352204
- NACRES NA.22
Safety
- Hazard Codes :Xn
- Risk Statements :22
- Safety Statements :24/25
- RIDADR :2811
- WGK Germany :3
- RTECS :US5625000
- F :10
- HazardClass :6.1(b)
- PackingGroup :III
- HS Code :29333999
- Hazardous Substances Data :536-69-6(Hazardous Substances Data)
- Toxicity :LD50 orally in mice: 230 mg/kg (Ishii)
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P264-P270-P301+P312-P501
FUSARIC ACID Price
More Price(4)
- Brand: Sigma-Aldrich(India)
- Product number: F6513
- Product name : Fusaric acid
- Purity: from Gibberella fujikuroi
- Packaging: 250MG
- Price: ₹9883.23
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: F6513
- Product name : Fusaric acid
- Purity: from Gibberella fujikuroi
- Packaging: 1G
- Price: ₹29617.2
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 55952
- Product name : Fusaric acid
- Purity: for HPLC derivatization, ≥99.0% (HPLC)
- Packaging: 1G
- Price: ₹30418.25
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: F0227
- Product name : 5-Butylpyridine-2-carboxylic Acid
- Purity: min. 98.0 %
- Packaging: 1G
- Price: ₹19800
- Updated: 2022/05/26
- Buy: Buy
FUSARIC ACID Chemical Properties,Usage,Production
- Chemical Properties off-white to faint yellowish crystalline powder
- Uses Fusaric acid may be used as a derivatizing reagent for the quantification of hydroxysteroids and dehydroepiandrosterone (DHEA)?and sulfated DHEA in biological samples using liquid chromatography electrospray-ionization-tandem mass spectrometry (LC/ESI-MS/MS) technique.
- Uses A medical research tool.
- Definition A mycotoxin and picolinic acid, that is an antibiotic and wilting agent that causes yellowing of infected plants.
- Synthesis Reference(s) The Journal of Organic Chemistry, 66, p. 605, 2001 DOI: 10.1021/jo0013554
- General Description Fusaric acid is a novel proton-affinitive derivatizing agent, having an ionization moiety and a hydrophobic moiety. It is commonly used for the derivatization of alcohols and phenols, by liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS).
- Hazard Very toxic.
- Biological Activity fusaric acid is a mycotoxin produced by several species of fusarium [1]. mycotoxins are biologically active secondary fungal metabolites found as contaminants of food- and feedstuff. mycotoxin is capable of causing disease and death in both humans and animals.fusaric acid is a potent inhibitor of dopamine β-hydroxylase. fusaric acid uncompetitively inhibited the activity of dopamine β-hydroxylase with an ic50 of 30 μm in an. fusaric acid lowered endogenous levels of norepinephrine and epinephrine in brain, spleen, heart, and adrenal glands. fusaric acid inhibited dopamine β-hydroxylase activity in adrenal medulla in vivo [2]. fusaric acid altered brain and pineal neurotransmitters. in the brain and pineal gland of rats, intraperitoneally (100 mg/kg) administration of fusaric acid increased the level of 5ht, 5-hydroxyindoleacetic acid (5hiaa), tyrosine, and dopamine [3].exposure to acute doses of fusaric acid caused vomiting and neurochemical changes in swine. fusaric acid might act synergistically with trichothecene mycotoxins to cause vomiting and feed refusal in pigs consuming trichothecene-contaminated feedstuffs [4].
- Safety Profile A poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. When heated to decomposition it emits toxic fumes of NOx.
- Purification Methods Dissolve it in CHCl3, dry (Na2SO4), filter, evaporate and recrystallise the residue from 50 parts of pet ether (b 40-60o), CHCl3/pet ether or EtOAc, then sublime it in vacuo. The amide crystallises from MeOH with m 128.2-129.0o. The copper salt forms bluish violet crystals from H2O and has m 258-259o. [Hardegger & Nikles Helv Chim Acta 39 505 1956, Schreiber & Adam Chem Ber 93 1848 1960, NMR and MS: Tschesche & Führer Chem Ber 111 3500 1978, Beilstein 22 III/IV 764, 22/2 V 384.]
-
References
[1] hidaka h, nagatsu t, takeya k, et al. fusaric acid, a hypotensive agent produced by fungi[j]. the journal of antibiotics, 1969, 22(5): 228-230.
[2] toshiharu n, hiroyoshi h, hiroshi k, et al. inhibition of dopamine β-hydroxylase by fusaric acid (5-butylpicolinic acid) in vitro and in vivo[j]. biochemical pharmacology, 1970, 19(1): 35-44.
[3] porter j k, bacon c w, wray e m, et al. fusaric acid in fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats[j]. natural toxins, 1995, 3(2): 91-100.
[4] smith t k, macdonald e j. effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine[j]. journal of animal science, 1991, 69(5): 2044-2049.
FUSARIC ACID Preparation Products And Raw materials
Raw materials
Preparation Products
Global(112)Suppliers
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-87569266<br/>15319487004
- Email:1015@dideu.com
- Country:China
- ProdList:3978
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
LEAPCHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:43340
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
536-69-6, FUSARIC ACIDRelated Search:
- 5-METHYLPICOLINIC ACID 3-BUTYLPYRIDINE 2-Picolinic acid 2-Hydrazinopyridine FUSARIC ACID 3-Ethylpyridine 2,5-Dimethylpyridine 5-Ethyl-2-methylpyridine 2-FORMYL-5-PICOLINE 2-Pyridinecarboxaldehyde 2-(Hydroxymethyl)pyridine 5-ETHYLPYRIDINE-2-CARBOXYLIC ACID 3-PROPYLPYRIDINE 5-BUTYL-2-METHYL-PYRIDINE CHEMBRDG-BB 4011741 PYRIDINIUM FORMATE fusaric acid N,N-diethylaminoethylamide RARECHEM AQ NN 0379
- 高端化学
- BioChemical
- Antibiotics A-F
- Antibiotics A to Z
- Antibiotics
- Dopamine beta-hydroxylase inhibitor.
- 乙腈中镰刀菌酸(萎蔫酸)溶液
- 镰刀菌酸/萎蔫酸标准品
- 镰刀菌酸/萎蔫酸
- 镰胞菌酸
- 镰刀菌酸
- 镰孢菌酸
- 萎蔫酸
- 5-丁基-2-吡啶羧酸
- 5-丁基-2-吡啶甲酸
- 芙莎酸
- 5-丁吡啶二甲酸
- 丁吡啶甲酸
- 5-丁基吡啶-2-羧酸
- 536-69-6
- 5-Butylpyridine-2-carboxylicAci
- 2-Pyridinecarboxylic acid, 5-butyl-
- 5-Butylpyridine-2-carboxylicAcid>
- NSC 135043
- Fusaric acid≥ 99.9% (assay)
- Fusaric Acid 5-Butylpicolinic Acid
- Fusaric acid, 99% 1GR
- Fusaric acid,99%
- 5-Butyl-2-pyridinecarboxylic acid, 5-Butylpicolinic acid, 5-Butylpyridine-2-carboxylic acid
- 5-Butylpicolinic acid, 5-Butylpyridine-2-carboxylic acid
- fusaric acid from gibberella fujikuroi
- Butylpicolinic acid
- Fusaric
- FUSARIC ACID 99%
- FUSARIC ACID DOPAMINE B-HYDROXYLAS
- 5-Buthyl picolinic acid
- 5-butylpyridine-3-carboxylic acid
- Picolinic acid, 5-butyl-
- fusarinicacid
- Fusarinic acid
- 5-n-butylpyridine-2-carboxylicacid
- 5-n-Butylpyridine-2-carboxylic acid
- 5-butyl-2-pyridinecarboxylicaci
- 5-BUTYLPYRIDINE-2-CARBOXYLIC ACID
- 5-BUTYLPICOLINIC ACID
- 5-BUTYL-2-PYRIDINECARBOXYLIC ACID
- 5-N-BUTYLPICOLINIC ACID
- 5-N-BUTYL-2-PICOLINIC ACID
- FURASIC ACID
- FUSARIC ACID