ChemicalBook > CAS DataBase List > MONOBEHENIN
MONOBEHENIN
MONOBEHENIN
- CAS No.30233-64-8
- Chemical Name:MONOBEHENIN
- CBNumber:CB4489612
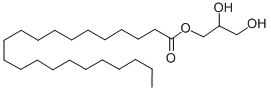
- Molecular Formula:C25H50O4
- Formula Weight:414.66
- MOL File:30233-64-8.mol
MONOBEHENIN Property
- Density 0.93[at 20℃]
- vapor pressure 0.001Pa at 25℃
- solubility Soluble, when heated, in chloroform and dichloromethane and in many organic solvents; slightly soluble in hot ethanol (96%); practically insoluble in cold ethanol (95%), hexane, mineral oil, and water.
- form A solid
- color White to off-white
- Water Solubility 100ng/L at 25℃
- LogP 8.58 at 25℃
- FDA UNII A626UU0W2A
- EPA Substance Registry System Docosanoic acid, monoester with 1,2,3-propanetriol (30233-64-8)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
MONOBEHENIN Chemical Properties,Usage,Production
- Chemical Properties Glyceryl behenate occurs as a fine white-yellow powder, as a hard waxy mass or pellet, or as white or almost white unctuous flakes. It has a faint odor.
- Definition ChEBI: 1-behenoylglycerol is a fatty acid ester resulting from the formal condensation of the hydroxy group at position-1 of glycerol with the carboxy group of docosanoic acid. It has a role as a plant metabolite and an antineoplastic agent. It is a 1-monoglyceride and a fatty acid ester. It is functionally related to a glycerol and a docosanoic acid.
- Production Methods Glyceryl behenate is prepared by the esterification of glycerin by behenic acid (C22 fatty acid) without the use of catalysts. In the case of Compritol 888 ATO (Gattefosse′), raw materials used are of vegetable origin, and the esterified material is atomized by spraycooling.
-
Pharmaceutical Applications
Glyceryl behenate is used in cosmetics, foods, and oral pharmaceutical
formulations.
In pharmaceutical formulations, glyceryl behenate is mainly used as a lubricant in the preparation of oral tablets and capsules. It has good binding properties, it does not affect tablet hardness and is unaffected by mixing or production parameters. Glyceryl behenate has been investigated for the encapsulation of various drugs such as retinoids. It has also been investigated for use in the preparation of sustained-release tablets; as a matrix-forming agent for the controlled release of water-soluble drugs; and it can also be used as a hot-melt coating agent sprayed onto a powder or drug-loaded sugar beads and granules. It may also be incorporated via extrusion/spheronization into pellets, which can be further compressed into tablets.
Glyceryl behenate is used in oral enteric-coated pellets, powders and suspensions. It is also used in controlled, extended-release and orally disintegrating tablets. For oral preparations, glyceryl behenate forms a lipidic matrix for sustained-release formulations. It has been used along with acid-soluble or swellable polymers to mask the bitter or unpleasant taste of the medicament with improved palatability.
Glyceryl behenate has been used for the preparation of ophthalmic inserts.
In cosmetics, glyceryl behenate is used as a skin conditioning agent, emollient and viscosity-increasing agent in emulsions. It also improves the heat stability of emulsions and is a gelifying agent for various oils. For topical formulations, it is used as a thickening agent for oily phases. It is also used as a surfactant or emulsifying agent. -
Safety
Glyceryl behenate is used in cosmetics, foods and oral pharmaceutical
formulations, and is generally regarded as a relatively
nonirritant and nontoxic material. The US Cosmetic Ingredients
Review Expert Panel evaluated glyceryl behenate and concluded
that it is safe for use in cosmetic formulations in present practices of
use and concentration.
LD50 (mouse, oral): 5 g/kg - storage Glyceryl behenate should be stored in a tightly closed container, at a temperature less than 35°C.
- Regulatory Status GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules, tablets, and suspensions). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
MONOBEHENIN Preparation Products And Raw materials
Raw materials
Preparation Products
Global(68)Suppliers
-
Supplier:
Jiangxi Alpha Hi-tech Pharmaceutical Co., Ltd
- Tel:510-85010237
- Email:overseamarketing@alphahi-tech.com
- Country:CHINA
- ProdList:41
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Shandong chuangyingchemical Co., Ltd.
- Tel:18853181302
- Email:sale@chuangyingchem.com
- Country:CHINA
- ProdList:5906
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Univar Solutions(China) Co., Ltd.
- Tel: +8615902132654
- Email:ivy.zhuang@univarsolutions.com
- Country:China
- ProdList:654
- Advantage:58
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12162
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
30233-64-8, MONOBEHENINRelated Search:
- TRITRICOSANOIN TRIBEHENIN 1-Myristin-3-Behenin? 1,2-Olein-3-Behenin? 1,3-BEHENIN-2-OLEIN 1,3-Tetracosanoin-2-Olein 1,2-Behenin-3-Myristin 1,3-DIBEHENIN MONOBEHENIN ETHYLHEXYLGLYCERYL BEHENATE GLYCERYL BEHENATE/EICOSADIOATE Glycerol Docosanoic acid 6,6'-DIBEHENOYL-ALPHA,ALPHA'-TREHALOSE 1,2-DIDOCOSANOYL-SN-GLYCERO-3-PHOSPHOCHOLINE 1-Myristin-2-Olein-3-Behenin URSODEOXYCHOLIC ACID ACYL-B-D-GLUCURONIDE DILIGNOCERIN
- 抑制剂
- 化妆品原料
- 甘油酯
- C25H50O4
- 二十二烷酸甘油酯,10 MM DMSO 溶液
- 单硬脂酸甘油酯杂质37
- 山嵛酸甘油酯对照品
- 二十二烷酸甘油酯
- 二十二酸-1,2,3-丙三醇单酯
- 甘油山嵛酸酯
- 二十二烷酸甘油单酯
- 30233-64-8
- Monobehenin, 10 mM in DMSO
- Monostearin Impurity 37
- Glycetyl Behenate
- Monobehenin,inhibit,Inhibitor,Bacterial
- Glyceryl Behenate (pharmaceutical grade)
- Behenic acid monoglyceride
- Docosanoicacidmonoesterwith1,2,3-propanetriol
- docosanoicacid,monoesterwith1,2,3-propanetriol
- MONOBEHENIN
- MONODOCOSANOIN
- 1-MONOBEHENIN
- KeMester 6500
- Docosanoin, Mono- (7CI)
- Docosanoic acid, Monoester with glycerol (8CI)
- Glyceryl monobehenate
- Docosansure, Monoester mit Glycerin
- NomINCI:Glyceryl(mono)behenate
- n-Docosanoicacidglycerolester
- docosanoic acid, monoester with glycerol