ChemicalBook > CAS DataBase List > Ibandronate sodium monohydrate
Ibandronate sodium monohydrate
Ibandronate sodium monohydrate
- CAS No.138926-19-9
- Chemical Name:Ibandronate sodium monohydrate
- CBNumber:CB4286017
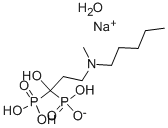
- Molecular Formula:C9H24NNaO8P2
- Formula Weight:359.23
- MOL File:138926-19-9.mol
Ibandronate sodium monohydrate Property
- Melting point 840C (dec)
- storage temp. Keep in dark place,Inert atmosphere,2-8°C
- solubility Soluble in DMSO (up to at least 25 mg/ml)
- form solid
- color White
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months.
- CAS DataBase Reference 138926-19-9(CAS DataBase Reference)
- FDA UNII J12U072QL0
- NCI Drug Dictionary Boniva
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :Xn
- Risk Statements :40
- Safety Statements :22-36-24/25
- HS Code :29319090
-
NFPA 704:
0 3 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
Ibandronate sodium monohydrate Chemical Properties,Usage,Production
-
Description
Ibandronate Sodium Monohydrate is a highly potent nitrogen-containing bisphosphonate used for the treatment of osteoporosis.
Ibandronate Sodium Monohydrate inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active and the biphosphonates are used in disorders affecting the skeleton such as metastatic disease, asteoporosis and Paget's. Ibandronate Sodium Monohydrate is an inhibitor of FDPS. - Chemical Properties [1-Hydroxy-3-(methylpentylamino)-propylidene]bisphosphonic acid sodium salt is White Crystalline Powder
- Uses Inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active. Biphosphonates are used in disorders affecting the skeleton such as asteoporosis, metastatic disease, and Paget d
- Uses An inhibitor of bone resorption
- Uses bone resorption inhibitor, anthypercalcemic
- Mechanism of action The action of ibandronate on bone tissue is based on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Ibandronate inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass.
-
Pharmacokinetics
Absorption
The absorption of oral ibandronate occurs in the upper gastrointestinal tract. Plasma concentrations increase in a dose-linear manner up to 50 mg oral intake and increases nonlinearly above this dose.
Following oral dosing, the time to maximum observed plasma ibandronate concentrations ranged from 0.5 to 2 hours (median 1 hour) in fasted healthy postmenopausal women. The mean oral bioavailability of 2.5 mg ibandronate was about 0.6% compared to intravenous dosing. The extent of absorption is impaired by food or beverages (other than plain water). The oral bioavailability of ibandronate is reduced by about 90% when BONIVA is administered concomitantly with a standard breakfast in comparison with bioavailability observed in fasted subjects. There is no meaningful reduction in bioavailability when ibandronate is taken at least 60 minutes before a meal. However, both bioavailability and the effect on bone mineral density (BMD) are reduced when food or beverages are taken less than 60 minutes following an ibandronate dose.
Distribution
After absorption, ibandronate either rapidly binds to bone or is excreted into urine. In humans, the apparent terminal volume of distribution is at least 90 L, and the amount of dose removed from the circulation via the bone is estimated to be 40% to 50% of the circulating dose. In vitro protein binding in human serum was 99.5% to 90.9% over an ibandronate concentration range of 2 to 10 ng/mL in one study and approximately 85.7% over a concentration range of 0.5 to 10 ng/mL in another study.
Metabolism
There is no evidence that ibandronate is metabolized in humans.
https://www.accessdata.fda.gov - References 1) Russell (2006), Ibandronate: Pharmacology and preclinical studies; Bone 38 S7
Ibandronate sodium monohydrate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(339)Suppliers
-
Supplier:
Chengdu Aslee Biopharmaceuticals, Inc.
- Tel:28-85305008
- Email:
- Country:CHINA
- ProdList:964
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19809
- Advantage:58
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Xiamen AmoyChem Co., Ltd
- Tel:+86-86-5926051114<br/>+8615060885618
- Email:sales@amoychem.com
- Country:China
- ProdList:6383
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
138926-19-9, Ibandronate sodium monohydrateRelated Search:
- Pamidronic acid Ibandronate Sodium Anhydrous Olpadronate Sodium b-Alanine, N-methyl-N-pentyl- Ibandronate Impurity 1(Ibandronate EP Impurity B) Alendronate sodium Letrozole 3,5-Bis(2-cyanoprop-2-yl)toluene INTERMEDIATE OF IBANDRONATE SODIUM N-Pentyl-β-alanine ETHYLENEDIAMINETETRAACETIC ACID TRIPOTASSIUM SALT DIHYDRATE Disodium edetate dihydrate N-DesMethyl Ibandronate SodiuM methyl N-(3-methoxy-3-oxopropyl)-N-methyl-beta-alaninate Tetramethyl Ibandronate Trisodium phosphate Acryloyl chloride N-Methylpentylamine
- Bone resorption inhibitor
- anti-osteoporosis
- Ibandronate
- API's
- Pharmaceuticals
- Intermediates & Fine Chemicals
- BONIVA
- Inhibitors
- 杂质对照品
- 药靶配体
- 化工原料
- 标准品
- 抑制剂
- 医药 抗骨质疏松
- 小分子抑制剂,天然产物
- 化学原药
- 医药原料药
- 原料
- 医药原料
- 抗骨质酥松
- 骨质疏松治疗
- 原料药
- C9H22NO7P2H2ONa
- C9H22O7NP2Na
- C9H22NNaO7P2H2O
- C9H22NO7P2NaH2O
- C9H22O7NP2NaH2O
- C9H22NO7P2Na
- C9H24NNaO8P2
- C9H24NO8NaP2
- C9H23NO7P2NaH2O
- 38926-19-9
- 伊班膦酸钠 / 伊班膦酸钠(一水)
- 1-羟基-3-(甲基戊基胺)-丙烷-1,1-双膦酸钠水合物
- 伊班磷酸钠一水合物
- 伊班膦酸钠水合物
- 伊班膦酸钠,1-羟基-3-(甲基戊基胺)-丙烷-1,1-双膦酸钠
- 伊班膦酸钠 USP标准品
- 伊班膦酸钠一水物/
- IBANDRONATE SODIUM, 中间体名 3-(N-METHYL-N-PENTYLAMINO)PROPIONIC ACID SODIUM SALT
- 1-羟基-3-(甲基戊基胺)-丙烷-1,1-双膦酸钠 一水合物
- 伊班膦酸钠单水合物
- 伊班磷酸钠
- 1-羟基-3-(甲基戊基胺)-丙烷-1,1-双膦酸钠(伊班膦酸钠)
- 伊班膦酸钠一水合物
- 138926-19-9
- Ibandronate sodium monohydrate - Bio-X ?
- Sodium hydrogen(1-hydroxy-3-(methyl(pentyl)amino)-1-phosphonopropyl)phosphonate hydrate
- Ibandronate Sodium (1335417)
- Ibandronate Sodium MonohydrateQ: What is Ibandronate Sodium Monohydrate Q: What is the CAS Number of Ibandronate Sodium Monohydrate Q: What is the storage condition of Ibandronate Sodium Monohydrate Q: What are the applications of Ibandronate Sodium Monohydrate
- [1-Hydroxy-3-(methylpentylamino)propylidene]bisphosphonic acid sodium salt monohydrate
- Ibronate Sodium
- RPR-102289A
- Boniva monohydrate
- Bondronat monohydrate
- Ibandronate sodium monohydrate, >=99%
- Ibandronate Sodium Hydrate
- Ibandronate Sodium (200 mg)