ChemicalBook > CAS DataBase List > DISULFOTON
DISULFOTON
DISULFOTON
- CAS No.298-04-4
- Chemical Name:DISULFOTON
- CBNumber:CB4208774
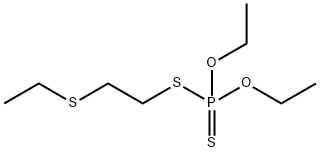
- Molecular Formula:C8H19O2PS3
- Formula Weight:274.4
- MOL File:298-04-4.mol
DISULFOTON Property
- Melting point 110-112℃
- Boiling point bp0.01 108°; bp1.5 132-133°
- Density 1.1445 g/cm3 (20 ºC)
- vapor pressure 7.2 x 10-3 Pa (20 °C)
- refractive index 1.5501 (589.3 nm 20℃)
- Flash point 133 °C
- storage temp. 0-6°C
- Water Solubility 25 mg l-1 (20 °C)
- form liquid
- Merck 13,3400
- BRN 1709167
- CAS DataBase Reference 298-04-4(CAS DataBase Reference)
- EWG's Food Scores 2
- FDA UNII 3CY5EKL6MT
- Pesticides Freedom of Information Act (FOIA) Disulfoton
- EPA Substance Registry System Disulfoton (298-04-4)
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :T+,N
- Risk Statements :27/28-50/53
- Safety Statements :28-36/37-45-60-61
- RIDADR :3018
- OEB :D
- OEL :TWA: 0.1 mg/m3 [skin]
- WGK Germany :3
- RTECS :TD9275000
- HazardClass :6.1(a)
- PackingGroup :I
- Hazardous Substances Data :298-04-4(Hazardous Substances Data)
- Toxicity :LD50 in female, male rats (mg/kg): 2.3, 6.8 orally; 6, 15 dermally (Gaines)
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H300+H310-H410
- Precautionary statements P262-P264-P273-P280-P301+P310-P302+P352+P310
DISULFOTON Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: 49784
- Product name : Disulfoton
- Purity: reference material
- Packaging: 100MG
- Price: ₹15306.55
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 45460
- Product name : Disulfoton
- Purity: PESTANAL?, analytical standard
- Packaging: 250MG
- Price: ₹7826.48
- Updated: 2022/06/14
- Buy: Buy
DISULFOTON Chemical Properties,Usage,Production
- Description Disulfoton is a combustible, colorless toyellowish oil with a characteristic odor. Technical productis a brown liquid. Molecular weight= 274.42; Boilingpoint= 132133℃ at 1.5 mm pressure; Freezing/Meltingpoint $ 225℃; Vapor pressure= 0.0002 mmHg at 20℃;Flash point=82℃. Hazard Identification (based on NFPA704 M Rating System): Health 4, Flammability 1,Reactivity 0. Practically insoluble in water;solubility 5 0.003% at 25℃
- Chemical Properties Disulfoton is a dark yellowish oil with an aromatic, sulfurous odor. It is soluble in most organic solvents and fatty oils. Disulfoton is a selective, systemic insecticide and acaricide. It is used for seed coating and for soil application to protect from insect attacks, for the control of sucking insects, aphids, leaf hoppers, thrips, beet-fl ies, spider mites, and coffeeleaf miners. Disulfoton has been used extensively in pest control on a variety of crops, such as cotton, tobacco, sugar beets, corn, peanuts, wheat, ornamentals, cereal grains, and potatoes. It is grouped by the US EPA under RUP. Human exposures to disulfoton occur through breathing contaminated air, drinking contaminated water, eating contaminated food, and working in industries that manufacture and formulate the pesticide.
- Uses Systemic insecticide and acaricide for control of sucking insects and mites in fruits, vegetables, cotton and forestry nurseries.
- Uses Disulfoton is used to control sucking insects and mites in a wide range of crops.
-
Uses
DISULFOTON is used as systemic insecticide and acaracide.
- Definition ChEBI: An organic thiophosphate that is the diethyl ester of S-[2-(ethylsulfanyl)ethyl] dihydrogen phosphorodithioate.
- General Description Disulphoton is a dark yellowish oil with an aromatic, sulphurous odour. It is soluble in most organic solvents and fatty oils. Disulphoton is a selective, systemic insecticide and acaricide. It is used for seed coating and for soil application to protect from insect attacks and for the control of sucking insects, aphids, leafhoppers, thrips, beetflies, spider mites, and coffee-leaf miners. Disulphoton has been extensively used in pest control on a variety of crops such as cotton, tobacco, sugar beets, corn, peanuts, wheat, ornamentals, cereal grains, and potatoes. It is grouped by the U.S. EPA under RUP. Human exposures to disulphoton occur through breathing contaminated air, drinking contaminated water, eating contaminated food, and working in industries that manufacture and formulate the pesticide.
- Reactivity Profile Organothiophosphates, such as DISULFOTON, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
- Health Hazard Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
- Health Hazard Disulfoton is highly toxic to animals and humans by all routes of exposures, namely, by dermal absorption, through ingestion, and inhalation by the respiratory route. The symptoms of poisoning include blurred vision, fatigue, headache, dizziness, sweating, tearing, and salivation. It inhibits cholinesterase and affects the nervous system function. It does not cause delayed neurotoxicity. Prolonged period of exposures to high concentrations of disulfoton cause harmful effects to the nervous system with symptoms such as narrowing of the pupils, vomiting, diarrhea, drooling, diffi culty in breathing, lung edema, tremors, convulsions, coma, and death. Disulfoton causes no mutagenic or teratogenic effects in laboratory animals. There are no reports indicating that disulfoton causes cancer in animals or humans. The DHHS, the IARC, and the US EPA have not classifi ed disulfoton as to its ability to cause cancer.
- Fire Hazard Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
- Agricultural Uses Insecticide, Acaricide: All products formulated at greater than 2% disulfoton are classified as Restricted Use Pesticides (RUP). Disulfoton is a selective, systemic organophosphate insecticide and acaricide that is especially effective against sucking insects. It is used to control aphids, leafhoppers, thrips, beet flies, spider mites, and coffee leaf miners. Not approved for use in EU countries. There are 21 global suppliers.
- Trade name BAY 19639®; BAYER 19639®; DIMAZ®; DISULFATON®; DI-SYSTON®[C]; DISYSTON®[C]; DISYSTOX®; DITHIODEMETON®; DITHIOSYSTOX®; EKATIN TD®; FRUMIN-AL®; FRUMIN G®; GLEBOFOS®; M-74®; S 276®; SOLVIREX®; THIODEMETON®; THIODEMETRON®
- Safety Profile Poison by ingestion, inhalation, skin contact, intraperitoneal, and intravenous routes. Human mutation data reported. When heated to decomposition it emits veq toxic SOx and POx. See also various demeton entries and ESTERS.
- Potential Exposure AgriculturalChemical; Mutagen; Human Data. Those involved in themanufacture, formulation, and application of this systemicinsecticide and acaricide.
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended following acuteoverexposure.
-
Environmental Fate
Soil/Plant. Disulfoton was metabolized in soil and plants to the corresponding sulfoxide and sulfone via oxidation of the thioether sulfur atoms (Metcalf et al., 1957; Getzin and Shanks, 1970; Takase et al., 1972; Clapp et al., 1976; Worthing and Hance, 1991), the corresponding phosphorothioate analogs and then to derivatives of O,O-diethyl hydrogen phosphate and 2-ethylthioethyl mercaptan (Worthing and Hance, 1991). Disulfoton is rapidly oxidized in soil to its sulfoxide and sulfone with disulfoton oxon sulfoxide and disulfoton oxon sulfone appearing in small amounts (Szeto et al., 1983). In a Portneuf silt loam soil, the persistence of the sulfoxide and sulfone was 32 and >64 days, respectively (Clapp et al., 1976).
Disulfoton was translocated from a sandy loam soil into asparagus tips. Disulfoton sulfoxide, disulfoton sulfone, disulfoton oxon sulfoxide and disulfoton oxon sulfone were recovered as metabolites (Szeto and Brown, 1982; Szeto et al., 1983). Disulfoton s
Groundwater. According to the U.S. EPA (1986) disulfoton has a high potential to leach to groundwater.
Photolytic. Disulfoton was rapidly oxidized to disulfoton sulfoxide and trace amounts (<5% yield) of disulfoton sulfone when sorbed on soil and exposed to sunlight (half-life 1–4 days) (Gohre and Miller, 1986). The photosensitized oxidation was probably due to the presence of singlet oxygen (Gohre and Miller, 1986; Zepp et al., 1981). The degradation rate was higher in soils containing the lowest organic carbon (Gohre and Miller, 1986). Chemical/Physical. Emits toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987; Lewis, 1990).
When fertilizers containing superphosphate and ammonium nitrate were impregnated with disulfoton, the latter chemically degraded to form disulfoton sulfone and disulfoton sulfoxide (Ibrahim et al., 1969).
The reported half-lives for abiotic hydrolysis - Metabolic pathway Disulfoton is metabolised by an analogous route to phorate. The principal route of disulfoton metabolism in all media is activation via oxidation of the thioether group to the sulfoxide (rapid) and sulfone (slower). Thioether oxidation occurs preferentially to oxidative desulfuration of the P=S group to the oxon, which is usually only present in trace amounts and there is good evidence that the sulfoxide and sulfone oxons arise via disulfoton sulfoxide and sulfone rather than disulfoton oxon. The more polar thiooxidised metabolites are translocated in plants and are responsible for the compound's systemic action.
- Metabolism The metabolic routes of disulfoton are essentially the same in plants, insects, and mammals, involving the oxidation of the sulfide group into the sulfoxide and then sulfone, oxidative desulfuration to the corresponding oxons, and hydrolysis to diethyl phosphorothioate. In mammals, orally administered disulfoton is rapidly metabolized and excreted in the urine. Disulfoton is rapidly degraded in soil; DT50 (20 ?C) was 1.3–2 d.
- storage Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with disulfotonyou should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilated
- Shipping Organophosphorus pesticides, solid, toxic, n.o.s.require a shipping label of “POISONOUS/TOXICMATERIALS.” They fall in DOT Hazard Class 6.1 andDisulfoton in Packing Group I.
- Degradation Disulfoton is stable at acidic and neutral pH values but it is hydrolysed in alkaline media.
- Toxicity evaluation The acute oral LD50 for rats is 2–12 mg/kg. Inhalation LC50 (4 h) for rats is 0.06–0.015 mg/L air. NOEL (2 yr) for rats is 1 mg/kg diet (0.05 mg/kg/d). ADI is 0.3 μg/kg b.w.
- Incompatibilities Contact with oxidizers may cause therelease of phosphorous oxides. Contact with strong reducingagents, such as hydrides, may cause the formation of flammable and toxic phosphine gas.
DISULFOTON Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
298-04-4, DISULFOTONRelated Search:
- DISULFOTON-OXON DISULFOTON-SULFONE,DISULFOTON SULFONE, 100MG,NEAT,DISULFOTON-SULFONE PESTANAL, 100 MG Dimethylphosphorodithioate DISULFOTON Diethylphosphorodithioate DISULFOTON-SULFOXIDE,Disulfoton disulide,DISULFOTON SULFOXIDE, 100MG, NEAT,DISULFOTON-SULFOXIDE PESTANAL, 100 MG,DISULFOTON-S THIOMETON 3,4-Dimethylphenol O,O,S-TRIMETHYLDITHIOPHOSPHATE DISULFOTON SULFONE DISULFOTON D10 (DI-ETHYL D5) DISULFOTON SULFOXIDE DISULFOTON-OXON-SULFOXIDE DISULFOTON SOLUTION 100UG/ML IN METHANOL 5ML DISULFOTON, 1X1ML, CH2CL2, 2000 UG/ML DISULFOTON SOLUTION 100UG/ML IN METHANOL 1ML DISULFOTON SOLUTION 100UG/ML IN METHANOL 5X1ML DISULFOTON-OXON-SULFON
- AcaricidesAlphabetic
- OrganophorousMore...Close...
- European Community: ISO and DIN
- Baby Food Directives 13/2003 EC&14/2003 ECPesticides&Metabolites
- AcaricidesMethod Specific
- Pesticides&Metabolites
- Pesticides
- Organophorous
- Insecticides
- EPA
- DAlphabetic
- D
- Alpha sort
- AcaricidesPesticides&Metabolites
- Endocrine Disruptors (Draft)Pesticides
- DIO - DIZMethod Specific
- 分析标准品
- 农残、兽药及化肥类
- 杀虫剂
- 有机氯杀虫剂
- C8H19O2PS
- 乙拌磷溶液标准物质
- 乙拌磷溶液
- 甲醇中乙拌磷溶液标准物质
- 甲醇中乙拌磷标准溶液
- 己烷/丙酮中乙拌磷标准溶液
- 乙腈中乙拌磷
- 乙拌磷检测标准品
- 甲醇中乙拌磷
- 乙拌磷标准溶液,100PPM
- 甲醇中乙拌磷溶液,1000ΜG/ML
- (剧毒,禁止销售)乙拌磷,100NG/UL溶于环己烷 标准品
- (剧毒,禁止销售)DISULFOTON,100NG/UL溶于乙腈 标准品
- 丙酮中乙拌磷标准品
- 丙酮中乙拌磷
- 乙拌磷溶液,100PPM
- 乙拌磷检测标样(100 ΜG/ML 溶剂为甲醇)
- 乙拌磷标准溶液
- 二硫酮
- 敌死通与异丙醇DISULFOTON&
- 双磺酸盐
- 二硫代磷酸-O,O-二乙基硫[2-(乙硫基)乙基]酯
- O,O-二乙基-S-2-(乙硫基)乙基二硫代磷酸酯
- 二硫代磷酸-O,O-二乙基-S-[2-(乙基硫代)乙基]酯(6CI,7CI,8CI,9CI)
- 二硫松
- 乙拌磷
- 敌死通
- 298-04-4
- Disulfoton Standard Solution
- Disulfoton Solution in Methanol, 1000μg/mL
- O,O-Diethyl 2-ethylthioethyl phosphorodithioate
- O,O-Diethyl S-(2-eththioethyl) phosphorodithioate
- O,O-Diethyl S-(2-eththioethyl) thiothionophosphate
- O,O-Diethyl S-(2-ethylmercaptoethyl) dithiophosphate
- o,o-Diethyl S-[2-(ethylsulfanyl)ethyl] dithiophosphate
- O,O-Diethyl S-[2-(Ethylthio)ethyl] dithiophosphate
- o,o-diethyl2-ethylthioethylphosphorodithioate
- O,O-DiethylO-[2-(ethylthio)ethyl]-dithiophosphate