ChemicalBook > CAS DataBase List > CARBINOXAMINE MALEATE SALT
CARBINOXAMINE MALEATE SALT
CARBINOXAMINE MALEATE SALT
- CAS No.3505-38-2
- Chemical Name:CARBINOXAMINE MALEATE SALT
- CBNumber:CB4183800
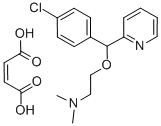
- Molecular Formula:C20H23ClN2O5
- Formula Weight:406.86
- MOL File:3505-38-2.mol
CARBINOXAMINE MALEATE SALT Property
- Melting point 116-1180C
- storage temp. Keep in dark place,Sealed in dry,Room Temperature
- Water Solubility Completely soluble in water
- solubility Chloroform: Slightly Soluble; Methanol: Slightly Soluble
- form Solid
- color White to Almost white
- Merck 14,1799
- InChIKey GVNWHCVWDRNXAZ-BTJKTKAUSA-N
- CAS DataBase Reference 3505-38-2(CAS DataBase Reference)
- FDA UNII 02O55696WH
- EPA Substance Registry System Ethanamine, 2-[(4-chlorophenyl)-2-pyridinylmethoxy]-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) (3505-38-2)
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :T
- Risk Statements :25-36/37/38
- Safety Statements :26-36/37/39-45
- RIDADR :3249
- WGK Germany :3
- RTECS :US6350000
- HazardClass :6.1(b)
- PackingGroup :III
- HS Code :2933399090
- Toxicity :LD50 in mice (mg/kg): 166 i.p. (Cahen)
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H301-H315-H319-H335
- Precautionary statements P301+P310+P330-P302+P352-P305+P351+P338
CARBINOXAMINE MALEATE SALT Price
More Price(4)
- Brand: Sigma-Aldrich(India)
- Product number: PHR2802
- Product name : Carbinoxamine Maleate
- Purity: certified reference material, pharmaceutical secondary standard
- Packaging: 400MG
- Price: ₹22342.8
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: C8278
- Product name : Carbinoxamine maleate salt
- Purity: analytical standard
- Packaging: 10G
- Price: ₹15717.9
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: C3057
- Product name : Carbinoxamine Maleate
- Purity:
- Packaging: 5G
- Price: ₹3900
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: C3057
- Product name : Carbinoxamine Maleate
- Purity:
- Packaging: 25G
- Price: ₹8000
- Updated: 2022/05/26
- Buy: Buy
CARBINOXAMINE MALEATE SALT Chemical Properties,Usage,Production
- Description Carbinoxamine is a competitive histamine H1 receptor antagonist (Ki = 2.3 nM) and first generation antihistamine. It also competes with [3H]diltiazem, an L-type calcium channel blocker, for binding to the benzothiazepine site on rat cardiomyocytes (Ki = 1.08 nM). Carbinoxamine decreases negative inotropic activity in isolated guinea pig left atria by 76% (EC50 = 250 nM) and negative chronotropic activity in guinea pig spontaneously beating isolated right atria (EC50s = 250 and 480 nM, respectively) by 48% compared with control. Formulations containing carbinoxamine have been used in the treatment of allergic rhinitis.
- Chemical Properties Off-White Solid
- Originator Clistin,McNeil,US,1953
- Uses antihistaminic
- Uses Analgesic and anti-inflammatory compound
- Definition ChEBI: The maleic acid salt of carbinoxamine. An ethanolamine-type antihistamine, used for treating hay fever, as well as mild cases of Parkinson's disease.
-
Manufacturing Process
As described in US Patent 2,800,485 a solution of p-chlorophenylmagnesium
bromide is prepared by adding dropwise a solution of 230 g (1.2 mols) of p-bromochlorobenzene in 900 cc of anhydrous ether to 26.7 g (1.1 g-atoms) of magnesium suspended in 100 cc of anhydrous ether containing a small crystal
of iodine. To this solution, 107 g (1 mol) of 2-pyridinealdehyde are added
slowly with stripping at a rate to maintain refluxing. The reaction mixture is
then stirred for one hour at room temperature. The mixture is then poured
onto an equal volume of crushed ice and water and acidified with concentrated
hydrochloric acid. The ether layer is removed. The aqueous layer is made
basic with ammonia and extracted with ether. The ether solution is evaporated
and the residue dried by addition of benzene and removal by distillation to
give 208 g (95%) of solid alpha-(p-chlorophenyl)-2-pyridinemethanol melting
at 78° to 80°C. The p-chlorophenyl pyridinemethanol may alternatively be
prepared from 4-chloroacetophenone, pyridine and granular aluminum as
described in US Patent 2,606,195. In either case, the synthesis then proceeds
as described in US Patent 2,800,485.
A solution of 219 g (1 mol) of α-(p-chlorophenyl)-2-pyridinemethanol in one liter of dry toluene is heated to 100°C with stirring. Twenty-three grams (1 g-atom) of sodium are then added in portions. After all the sodium has reacted, a dried solution of 2-dimethylaminoethyl chloride in benzene is added. This benzene solution is prepared by dissolving 173 g (1.2 mols) of 2- dimethylaminoethyl chloride hydrochloride in the minimum amount of water, adding 500 cc of benzene followed by 300 g of sodium carbonate decahydrate, stirring, separating the benzene layer and drying.
The mixture is refluxed with stirring for ten hours, cooled and filtered. The filtrate is extracted three times with 200 cc portions of 6 N acetic acid. The aqueous acetic acid solution is then made strongly basic with 10% sodium hydroxide solution, and extracted three times with 200 cc portions of ether. The ether extract is dried with anhydrous sodium sulfate, stirred with 5 g of activated carbon and filtered to provide 2-[p-chloro-α(2-dimethylaminoethoxy) benzyl]pyridine in solution. Addition of a solution of 116 g (1 mol) of maleic acid in 1,500 cc of ether gives 323 g (79%) of solid which, on recrystallization from ethyl acetate, gives white solid 2-[p-chloro-α(2-dimethylaminoethoxy) benzyl]pyridine maleate melting at 117° to 119°C. - brand name Clistin (Ortho- McNeil).
- Therapeutic Function Antihistaminic
-
General Description
Carbinoxamine is availableas a bitter bimaleate salt, (d, l)-2-[p-chloro- -[2-(dimethylamino)ethoxy]benzyl]pyridine bimaleate (Clistin), which isa white crystalline powder that is very soluble in water andfreely soluble in alcohol and in chloroform. The pH of a 1%solution is between 4.6 and 5.1.
Carbinoxamine is a potent antihistaminic and is availableas the racemic mixture. It differs structurally from chlorpheniramineonly in having an oxygen atom separate theasymmetric carbon atom from the aminoethyl side chain. Themore active levo isomer of carbinoxamine has the (S) absoluteconfiguration and can be superimposed on the moreactive dextro isomer (S configuration) of chlorpheniramine.
CARBINOXAMINE MALEATE SALT Preparation Products And Raw materials
Raw materials
Preparation Products
Global(216)Suppliers
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
Anhui Ruihan Technology Co., Ltd
- Tel: +8617756083858
- Email:daisy@anhuiruihan.com
- Country:China
- ProdList:973
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12186
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Xi'an MC Biotech, Co., Ltd.
- Tel:029-89275612<br/>+8618991951683
- Email:mcbio_sales@163.com
- Country:China
- ProdList:2251
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
3505-38-2, CARBINOXAMINE MALEATE SALTRelated Search:
- CLISTIN
- Heterocycles
- Aromatics
- Neat Compounds
- Drugs&Metabolites
- CA - CGForensic and Veterinary Standards
- C
- Alphabetic
- Pharmaceuticals
- Intermediates & Fine Chemicals
- 高纯化学试剂
- 抑制剂
- 化学试剂
- 化工中间体-化学试剂
- 药靶配体
- 医用原料
- 马来酸卡比沙明系列产品
- 医药原料
- 000-中间体
- 抗过敏免疫遗传类药
- 优势产品
- 医药原料药-科研原料
- 医药原料
- 合成
- 小分子抑制剂
- Analytical Standards
- Alphabetic
- Analytical Chromatography Product Catalog
- CA - CG
- C20H23ClN2O5
- C17H22N2OC4H4O4
- C16H19ClN2OC4H4O4
- CARBINOXAMINE MALEATE 马来酸罗托沙敏 |CAS 3505-38-2
- 马来酸罗托沙敏,10 MM DMSO 溶液
- LERGEFIN|||马来酸罗托沙敏|||CARBINOXAMINE MALEATE
- 马来酸卡宾诺沙明
- 马来酸氯苯吡醇胺
- (2-((4-氯苯基)(2-吡啶基)甲氧基)乙基)-N,N-二甲基胺马来酸盐
- 马来酸罗托沙敏杂质
- N-[2-[(4-氯苯基)(2-吡啶基)甲氧基]乙基]-N,N-二甲基胺马来酸盐
- 马来酸卡比沙明、马来酸罗托沙敏
- 卡比沙明 马来酸盐
- {2-[(4-氯苯基)(吡啶-2-基)甲氧基]乙基}二甲胺马来酸盐
- 马来酸卡比沙明
- 马来酸罗托沙敏
- 2-[(4-氯苯基)-(2-吡啶基)甲氧基]乙基-二甲基-胺
- 卡比沙明氢马来酸盐
- 对-马来酸罗托沙敏
- 对马来酸罗托沙敏
- 丁-2-烯二酸
- 3505-38-2
- Carbinoxamine Maleate Salt, 10 mM in DMSO
- Lergefin|||CarbinoxaMine Maleate
- Carbinoxamine Maleate, ≥ 98.0%
- Carbinoxamine Maleate (1096000)
- "(+/-)-Carbinoxamine D6 Maleate (N,N-dimethyl-d6)"
- CARBINOXAMINE MALEATE SALT USP/EP/BP
- But-2-enedioic acid (2-[(4-chlorophenyl)(pyridin-2-yl)methoxy]ethyl)dimethylamine