ChemicalBook > CAS DataBase List > Eradicane
Eradicane
Eradicane
- CAS No.759-94-4
- Chemical Name:Eradicane
- CBNumber:CB4169538
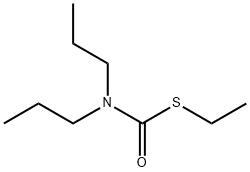
- Molecular Formula:C9H19NOS
- Formula Weight:189.32
- MOL File:759-94-4.mol
Eradicane Property
- Melting point 25°C
- Boiling point 127°C (20 torr)
- Density 0.95
- vapor pressure 3.192Pa at 25℃
- refractive index nD30 1.4750
- Flash point 116 °C
- storage temp. 0-6°C
- solubility Chloroform (Slightly), Methanol (Slightly)
- pka -1.22±0.70(Predicted)
- form Liquid
- color Clear colorless
- Water Solubility 0.375 g/100 mL
- Merck 13,3669
- BRN 1762751
- Stability Stable. Incompatible with strong oxidizing agents.
- LogP 3.21 at 20℃
- EWG's Food Scores 1
- FDA UNII R7PI3287F4
- Proposition 65 List Ethyl Dipropylthiocarbamate
- NIST Chemistry Reference Carbamothioic acid, dipropyl-, s-ethyl ester(759-94-4)
- EPA Substance Registry System S-Ethyl dipropylthiocarbamate (759-94-4)
- Pesticides Freedom of Information Act (FOIA) Eptam
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :Xn
- Risk Statements :22
- Safety Statements :2-23
- RIDADR :2902
- WGK Germany :1
- RTECS :FA4550000
- HazardClass :6.1(b)
- PackingGroup :III
- HS Code :29302000
- Hazardous Substances Data :759-94-4(Hazardous Substances Data)
- Toxicity :LD50 orally in rats: 1631 mg/kg (Antognini)
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H302+H312-H315-H317-H319-H331-H411
- Precautionary statements P273-P280-P301+P312-P302+P352+P312-P304+P340+P311-P305+P351+P338
Eradicane Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 45469
- Product name : EPTC
- Purity: PESTANAL?, analytical standard
- Packaging: 250MG
- Price: ₹6516.65
- Updated: 2022/06/14
- Buy: Buy
Eradicane Chemical Properties,Usage,Production
- Chemical Properties Light yellow liquid or yellow granular material. Commercial products may be in the form of dusts, sprays, solutions, wettable powder suspensions or emulsions.
- Uses A preemergence herbicide.
- Uses EPTC is a pesticide, S-Ethyl-N,N-dipropylthiocarbamate.
- Uses Selective systemic herbicide used for preemergence control of perennial and annual grasses such as johnsongrass, nutgrass and quackgrass. Also for control of some broadleaved weeds such as chickweed, henbit, lambsquarters, pigweed and purslane. EPTC is also used in vegetable crops, alfalfa, cotton, flax, pineapple, almonds and walnuts.
- Definition ChEBI: EPTC is a tertiary amine.
- Synthesis Reference(s) Journal of the American Chemical Society, 81, p. 714, 1959 DOI: 10.1021/ja01512a052
-
General Description
S-Ethyl dipropylthiocarbamate (EPTC) is a pale to dark yellow liquid with an aromatic odour/pleasant scent characteristic to thiocarbamates. EPTC is a commonly used herbicide. EPTC has a pleasant scent and at 20°C is miscible with most organic solvents, including acetone, ethyl alcohol, kerosene, methyl isobutyl ketone (MIBK), and xylene.
EPTC was the first thiocarbamate herbicide developed. EPTC formulations are used for the control of preemergent weeds, especially grasses. EPTC inhibits photosynthesis, respiration, and the synthesis of lipids, proteins, and RNA in these seedlings. Resistant plants are less sensitive to the herbicidal action apparently due to their ability to rapidly metabolise EPTC. - Air & Water Reactions Water insoluble. Slowly decomposes in water to form carbon disulfide and propyl amine. Such decompositions are accelerated by acids.
- Reactivity Profile Eradicane may generate flammable gases with aldehydes, nitrides, and hydrides. Incompatible with acids, peroxides, and acid halides.
- Health Hazard SYMPTOMS: Symptoms of exposure to Eradicane may include headache, giddiness, nervousness, blurred vision, weakness, nausea, cramps, diarrhea, sweating, miosis, tearing, salivation, vomiting and cyanosis.
- Fire Hazard Flash point data are not available for Eradicane, but Eradicane is probably combustible.
- Flammability and Explosibility Non flammable
- Agricultural Uses Herbicide: Some formulations are Restricted Group Pesticides (RUP). EPTC is a pre-emergence and early post-emergence herbicide used to control the growth of germinating annual weeds, including broadleaves, grasses, and sedges. It is used in every region of the United States in the production of a wide variety of food crops. The heaviest usage is in the Corn Belt, Northeastern and Mid-Atlantic states, Coastal and Northern Great Plains and in the Pacific Northwest on corn, potatoes, sweet potatoes, dry beans, peas, alfalfa, and snap beans. EPTC is also used on home-grown vegetables and ornamentals. Not approved for use in EU countries. Registered for use in the U.S. and other countries
- Trade name ALIROX®; EPTAM®; EPTAM® 6E; EPTAM 2.3G (granular, 2.3% by weight); EPTAM 10G (granular, 10% by weight); ERADICANE®; GENEP® EPTC; R-1608®; SHORTSTOP®; STAUFFER® R 1608; TORBIN®
- Safety Profile Poison by ingestion, inhalation, and intravenous routes. Moderately toxic by skin contact route. Mutation data reported. An herbicide. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also CARBAMATES
- Potential Exposure EPTC is a pre-emergence and early postemergence thiocarbamate herbicide used to control the growth of germinating annual weeds, including broadleaves, grasses, and sedges. It is used in every region of the United States in the production of a wide variety of food crops. The heaviest usage is in the Corn Belt, Northeastern and Mid-Atlantic states, Coastal and Northern Great Plains and in the Pacific Northwest on corn, potatoes, sweet potatoes, dry beans, peas, alfalfa, and snap beans. EPTC is also used on home-grown vegetables and ornamentals. Some formulations are Restricted Group Pesticides (RUP)
-
Environmental Fate
Soil. EPTC is rapidly degraded by soil microbes and fungi yielding carbon dioxide,
ethyl mercaptan and amino residues (Kaufman, 1967; Lee, 1984; Hartley and Kidd, 1987).
Degradation occurs via hydrolysis of the ester linkage forming the corresponding mercaptan,
alkylamine (n-dipropylamine) and carbon dioxide. Transthiolation and oxidation of
the mercaptan forms the alcohol which is further oxidized to afford a metabolic pool
(Kaufman, 1967). EPTC partially degraded in both sterile and nonsterile clay soils. Mineralization
was not observed since carbon dioxide was not detected (MacRae and Alex-
ander, 1965). EPTC sulfoxide was also reported as a metabolite identified in soil (Somasundaram
and Coats, 1991) and in corn plants (Casida et al., 1974). The rapid formation
of carbon dioxide was also observed from the microbial degradation of EPTC by a
microbial metabolite isolated from Jimtown loam soil and designated JE1 (Dick et al.,
1990). These researchers proposed that EPTC hydroxylated at the a-propyl carbon forming
the unstable a-hydroxypropyl EPTC which degrades to N-depropyl EPTC and propionaldehyde.
Metabolization of N-depropyl EPTC yields s-ethyl formic acid and propylamine.
Demethylation of s-ethyl formic acid gives s-methyl formic acid. Propylamine and smethyl
formic acid probably degrades to ammonia and methyl mercaptan, respectively
and carbon dioxide (Dick et al., 1990). The reported half-lives in soil ranged from 2 to 4
weeks in two agricultural soils (Regina heavy clay and Weyburn loam) (Smith and Fitzpatrick,
1970) to 30 days (Jury et al., 1987). Rajagopal et al. (1989) reported that the
persistence of EPTC in soil ranged from <4 to 6 weeks when applied at recommended rates.
Plant. EPTC is rapidly metabolized by plants to carbon dioxide and naturally occurring plants constituents (Humburg et al., 1989).
Chemical/Physical. Emits toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987).
In the gas phase, EPTC reacts with hydroxyl and/or NO3 radicals but not with ozone. With hydroxy radicals in the presence of NO3, S-ethyl N-formyl-N-propylthiocarbamate formed as the major product. With NO3 radicals only, the major product was C3H7(CHO)NC(O)SC2H5. In addition, a minor product formed tentatively identified as CH3CH2CH2(CH3CH2C(O))NC(O)SCH2CH3. The calculated photolysis lifetimes of EPTC in the troposphere with hydroxyl and NO3 radicals are 5.8 hours and 5.0 days, respectively (Kwok et al., 1992). - Metabolic pathway A new class of xenobiotic metabolite derived from the glutathione conjugate is produced by corn, cotton, and corn cell suspension cultures treated with 14C-EPTC (S-ethyl dipropylthiocarbamate). This metabolite is characterized as S-(N,N-dipropylcarbamoyl)-O- malonyl-3-thiolactic acid and is not detected in soybean plants or peanut cell suspension cultures. A second major metabolite of EPTC, S-(N,N- dipropylcarbamoyl)-N-malonylcysteine, is present in all tissues.
- Shipping UN2902 Pesticides, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
- Incompatibilities Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Thermal decomposition of thiocarbamate compounds include carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, andmethylamine. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.
- Waste Disposal Land burial is acceptable for small quantities. Larger quantities can be incinerated. (EPTC is combustible and could be incinerated. Recommendable method: Incineration. Peer-review: Incineration in a unit witheffluent gas scrubbing is recommendable for large amount. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Eradicane Preparation Products And Raw materials
Raw materials
Preparation Products
Global(118)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Xiamen AmoyChem Co., Ltd
- Tel:+86-86-5926051114<br/>+8615060885618
- Email:sales@amoychem.com
- Country:China
- ProdList:6383
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ShanDong Look Chemical Co.,Ltd.
- Tel: +8617653113219
- Email:sales01@sdlookchemical.com
- Country:China
- ProdList:2737
- Advantage:58
-
Supplier:
LEAP CHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:24727
- Advantage:58
759-94-4, EradicaneRelated Search:
- Pesticides&Metabolites
- EQ - EZ
- E-GAlphabetic
- E
- Alpha sort
- 高端化学
- 农药中间体
- 分析标准品
- 农残、兽药及化肥类
- 挥发性有机化合物(VOCs)
- 农药
- 除草剂
- 氨基甲酸酯类除草剂
- C9H19NOS
- 759-94-1
- 甲苯中茵草敌
- S-乙基二丙基硫代氨基甲酸酯
- 丙草丹-茵草敌
- 茵草敌 标准品
- 10NG/UL,10ML菌草敌溶于环己烷中 标准品
- EPTC@1000 ΜG/ML IN METHANOL
- 甲苯中茵草敌标准品
- 菌达灭
- S-乙基-N,N-二正丙基硫代氨基甲酸酯
- 苯基硫代氨基甲酸乙酯/丙草丹
- 扑草灭
- 茵达灭
- 茵草敌
- N,N-二丙基硫赶氨基甲酸S-乙酯
- N,N-二丙基硫代氨基甲酸-S-乙基酯
- 茵草敌溶液, 100PPM
- 丙草丹/茵达灭
- 丙草丹标准溶液
- S-乙基-N,N-二丙基硫代氨基甲酸酯
- 苯基硫代氨基甲酸乙酯/丙草丹/茵草敌
- 苯基硫代氨基甲酸乙酯
- S-乙基二正丙基硫代氨基甲酸酯
- 丙草丹
- 759-94-4
- Phenyl Acetate Impurity 10
- L13190000CY EPTC10μg/mLin Cyclohexane
- EPTC 100 μg/ml Acetonitrile
- C13190000 EPTC
- EPTC(s-ethyldipropylthiocarbamate)Solution,1,000mg/L,1ml
- EPTC@1000 μg/mL in Methanol
- Carbamothioic acid, N,N-dipropyl-, S-ethyl ester
- ethyldi-n-propylthiolcarbamate
- Ethyl N,N-dipropylthiolcarbamate
- Ethyl N,N-di-n-propylthiolcarbamate
- Ethyl di-n-propylthiolcarbamate
- EPTC (pesticide, MW 189)
- eptam6e
- Eptam 6E
- dipropylthio-carbamicacis-ethylester
- dipropylthiocarbamicacids-ethylester
- dipropyl-carbamothioicacis-ethylester
- dipropylcarbamothioicacids-ethylester
- Dipropylcarbamothioic acid S-ethyl ester