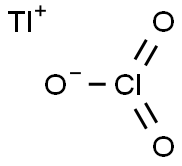ChemicalBook > CAS DataBase List > Thallium chlorate
Thallium chlorate
Thallium chlorate
- CAS No.13453-30-0
- Chemical Name:Thallium chlorate
- CBNumber:CB3851803
- Molecular Formula:ClO3Tl
- Formula Weight:287.8345
- MOL File:13453-30-0.mol
Thallium chlorate Property
- Density 5.500
- form colorless hexagonal crystals
- color colorless hexagonal, hexane crystals, crystalline
- Water Solubility g/100g H2O: 2.00 (0°C), 3.92 (20°C), 57.3 (100°C) [LAN05]
- EPA Substance Registry System Chloric acid, thallium(1+) salt (13453-30-0)
Safety
- RIDADR :2573
- HazardClass :5.1
- PackingGroup :II
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Thallium chlorate Chemical Properties,Usage,Production
- Chemical Properties needles [LAN05]
- General Description A white crystalline solid. Denser than water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
- Air & Water Reactions Slightly soluble in cold water; soluble in hot water.
- Reactivity Profile Thallium chlorate is an oxidizing agent. May initiate combustion when exposed to organic materials. May liberate explosive chlorine dioxide gas when heated in the presence of strong acid. May liberate chlorine dioxide and carbon dioxide if heated in contact with moist dibasic carboxylic acids. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806]. A combination with finely divided aluminum may explode by heat, percussion, or friction [Mellor 2:310 1946-47].
- Health Hazard Toxic by ingestion. Inhalation of dust is toxic. Fire may produce irritating, corrosive and/or toxic gases. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
- Fire Hazard These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
- Safety Profile A poison and oxidner. When heated to decomposition it emits toxic fumes of T1 and Cl-.
- Purification Methods It crystallises from hot water (2mL/g) on cooling. Its solubility in H2O (w/w) is 0.17% at 0o, 0.32% at 20o, and 2.4% at 100o. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 870 1963]. POISONOUS.
Thallium chlorate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(6)Suppliers
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8772
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39927
- Advantage:58
- Supplier: Mainchem Co., Ltd.
- Tel:+86-0592-6210733
- Email:sale@mainchem.com
- Country:China
- ProdList:32343
- Advantage:55
13453-30-0, Thallium chlorateRelated Search:
- 氯酸铊
- 氯酸亚铊
- 13453-30-0
- thallium(1+),chlorate
- Thallium(I)-chlorat
- Thallium chlorate
- Thallous chlorate