ChemicalBook > CAS DataBase List > Aluminum Bromide
Aluminum Bromide
Aluminum Bromide
- CAS No.7727-15-3
- Chemical Name:Aluminum Bromide
- CBNumber:CB3464331
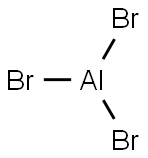
- Molecular Formula:AlBr3
- Formula Weight:266.69
- MOL File:7727-15-3.mol
Aluminum Bromide Property
- Melting point 94-98 °C(lit.)
- Boiling point 265 °C
- Density 3.205 g/mL at 25 °C(lit.)
- vapor pressure 1 mm Hg ( 81.3 °C)
- Flash point 268°C subl.
- storage temp. 2-8°C
- solubility Soluble in benzene, nitrobenzene, toluene, xylene, ether, simple hydrocarbons, alcohol, carbon disulfide, ether.
- form powder
- color White to orange
- Specific Gravity 2.64
- Water Solubility decomposes
- Sensitive Moisture Sensitive
- Merck 14,332
- Dielectric constant 3.4(100℃)
- Stability Stable, but reacts violently with water. Incompatible with aqueous solutions, alcohols, acids.
- InChIKey PQLAYKMGZDUDLQ-UHFFFAOYSA-K
- CAS DataBase Reference 7727-15-3(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII IY20HBK5LK
- NIST Chemistry Reference Aluminum tribromide(7727-15-3)
- EPA Substance Registry System Aluminum bromide (AlBr3) (7727-15-3)
- UNSPSC Code 12352302
- NACRES NA.23
Safety
- Hazard Codes :C,Xi,N,F
- Risk Statements :23/24/25-34-22-14-67-65-50/53-11-52/53-20
- Safety Statements :9-16-26-29-36/37/39-43-45-60-61-62-28-27
- RIDADR :UN 3264 8/PG 2
- WGK Germany :3
- RTECS :BD0350000
- F :21
- Hazard Note :Irritant
- TSCA :Yes
- HazardClass :8
- PackingGroup :II
- HS Code :28275900
- Hazardous Substances Data :7727-15-3(Hazardous Substances Data)
- Toxicity :LD50 orally in Rabbit: 1598 mg/kg
-
NFPA 704:
0 3 0 W
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H290-H302-H314
- Precautionary statements P234-P260-P280-P301+P312-P303+P361+P353-P305+P351+P338
Aluminum Bromide Price
More Price(17)
- Brand: Sigma-Aldrich(India)
- Product number: 8.01068
- Product name : Aluminium bromide
- Purity: anhydrous for synthesis
- Packaging: 25G
- Price: ₹6330.01
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.01068
- Product name : Aluminium bromide
- Purity: anhydrous for synthesis
- Packaging: 100G
- Price: ₹17810.01
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.01068
- Product name : Aluminium bromide
- Purity: anhydrous for synthesis
- Packaging: 8010682500
- Price: ₹402389.99
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 449601
- Product name : Aluminum bromide
- Purity: anhydrous, powder, 99.999% trace metals basis
- Packaging: 5G
- Price: ₹10456.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 401218
- Product name : Aluminum bromide
- Purity: ≥99.99% trace metals basis
- Packaging: 5G
- Price: ₹4449.08
- Updated: 2022/06/14
- Buy: Buy
Aluminum Bromide Chemical Properties,Usage,Production
- Description Aluminum bromide is a light-yellow colored liquid or colorless monoclinic crystals. Very corrosive to skin, eyes and mucous membranes, moderately poisonous. Aluminum bromide is an inorganic binary compound. The substance is a salt of aluminum and bromine-hydrogen acid. it exists in form of a dimer. The commercial use of aluminum bromide is relatively insignificant at the moment. Aluminum bromide belongs as the main component to xylene electrolytes for electric precipitation of aluminum coatings. The anhydrous aluminum bromide is used in organic synthesis, in particular, in the alkylation reaction or Friedel-Crafts reaction by analogy with aluminum chloride.This compound can be catalyst in a bromo-alkanes` isomerizating reaction. Aluminum bromide can also be used as bromating agent, for example, in a reaction with chloroform.
- Uses The anhydrous form is used as a catalyst for the Friedel-Crafts alkylation reaction. Its catalytic activity is similar to anhydrous AlCl3. Commercial applications, however, are few.
-
Preparation
Prepared from bromine and metallic aluminum.
2Al + 3Br2 →Al2Br6 (anhydrous) -
Reactions
Decomposes upon heating in air to bromine and metallic aluminum.
2 AlBr3→ 2Al + 3Br2
Reacts with carbon tetrachloride at 100°C to form carbon tetrabromide;
4AlBr3 + 3CCl4 → 4AlCl3 + 3Br4
Reaction with phosgene yields carbonyl bromide and aluminum chlorobromide;
AlBr3 + COCl2 → COBr2 + AlCl2Br
Reacts violently with water; absorbs moisture forming hexahydrate, AlBr3⋅6H2O - Chemical Properties White Crystalline Powder
- Physical properties Colorless crystalline solid in anhydrous form; melts at 97.5°C; boils at 256°C;density 3.01 g/cm3 at 25°C; moisture sensitive, fumes in air; soluble in water (reacts violently in cold water, and decomposes in hot water, alcohols, acetone, hexane, benzene, nitrobenzene, carbon disulfide and many other organic sol_x0002_vents).
- Uses Anhydrous: bromination, alkylation, and isomerization catalyst in organic synthesis.
- Uses Alkylation, bromination and isomerization catalyst in organic synthesis.Aluminum bromide acts as a strong Lewis acid and is used in Lewis acid-promoted reactions such as epoxide ring openings. It is used as a catalyst for Friedel-Crafts alkylation reaction. It also finds application in bromination and isomerization reactions in organic synthesis. It is also used in polymerization reaction to prepare poly(o-xylene)from benzocyclobutene.
-
Preparation
Prepared from bromine and metallic aluminum.
2Al + 3Br2 ——? Al2Br6 (anhydrous). - General Description A white to yellowish-red, lumpy solid with a pungent odor.
- Reactivity Profile An acid. May catalyze organic reactions. Corrosive to metals. Solutions of aluminum bromide in dichloromethane should be kept cold as a potentially dangerous exothermic halide exchange reaction occurs on warming, [Acc. Chem. Res., 1986, 19(3), 78].
- Hazard The anhydrous form reacts violently with water; corrosive to skin.
- Health Hazard CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
- Fire Hazard EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
-
reaction suitability
reagent type: catalyst
core: aluminum - Safety Profile A toxic, corrosive material. See also BROMIDES and ALUMINUM COMPOUNDS. Mixtures with sodium or potassium explode violently upon impact. When heated to decomposition it emits toxic fumes of Br-. Do not add H2O to anhydrous material. Hydrolysis can be violent.
- Purification Methods Reflux it and then distil it from pure aluminium chips in a stream of nitrogen into a flask containing more of the chips. It is then distilled under vacuum into ampoules [Tipper & Walker J Chem Soc 1352 1959]. Anhydrous conditions are essential, and the white to very light brown solid distillate can be broken into lumps in a dry-box (under nitrogen). It fumes in moist air. [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 812-813 1963.]
Aluminum Bromide Preparation Products And Raw materials
Raw materials
Preparation Products
Global(201)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Richest Group Ltd
- Tel:18017061086
- Email:oled@richest-group.com
- Country:CHINA
- ProdList:5600
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15416
- Advantage:58
-
Supplier:
Baoji Guokang Healthchem co.,ltd
- Tel:+8615604608665<br/>15604608665
- Email:dominicguo@gk-bio.com
- Country:CHINA
- ProdList:9414
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Shandong Zhishang New Material Co., Ltd.
- Tel: +8617653113209
- Email:sales002@sdzschem.com
- Country:China
- ProdList:3049
- Advantage:58
7727-15-3, Aluminum BromideRelated Search:
- Potassium bromide Phosphorus tribromide Cuprous bromide Boron tribromide Tetrabutylammonium bromide Calcium bromide Ammonium bromide Clidinium bromide Sodium bromate Sodium bromide Ethidium bromide Trimethylsulfonium bromide Benzyl bromide Hydrogen bromide NICKEL BROMATE CADMIUM BROMIDE TETRAHYDRATE Cesium bromide Lithium Bromide hydrate Aluminium Tribromide
- metal halide
- Inorganics
- Salts
- Lewis Acids
- Crystal Grade Inorganics
- Chemical Synthesis
- Catalysis and Inorganic Chemistry
- AluminumSynthetic Reagents
- AluminumMetal and Ceramic Science
- Aluminum Salts
- 无机化工原料
- 高端化学
- 格氏试剂
- 通用试剂
- 原料
- 催化剂
- 中间体
- 无机卤化物
- 无机化工
- 无机盐
- 无机化工产品
- Cc溴化合物
- 催化和无机化学
- 轻金属
- 铝
- Aluminum
- Synthetic Reagents
- Lewis Acids
- A1Br3
- AlBr36H2O
- 溴化铝(六水)
- 溴化铝 溶液
- 溴化铝, 无水
- 溴化铝, 超干, 99.999% (METALS BASIS)
- 溴化铝, ULTRA DRY, 99.999% (METALS BASIS)
- 溴化铝,98%
- 溴化铝, 半干, 99.999% (METALS BASIS)
- 溴化铝, 99.997% (METALS BASIS)
- 无水溴化铝
- 无水三溴化铝
- 三溴化铝 无水
- 三溴化铝(无水)
- X**溴化铝
- 7727-15-3?三溴化铝
- 六水合溴化铝
- 溴化鋁
- 溴化铝,半干
- 溴化铝
- 三溴化铝
- 7727-15-3
- AlBr3
- Aluminium tribromide
- ALUMINIUM BROMIDE
- Aluminum bromide, White to orange crystalline, 98%,
- Aluminum Bromide ISO 9001:2015 REACH
- uminum bromide
- AluminumBromideAnhydride>
- Aluminium(III) bromide, anhydrous powder 99.999%