ChemicalBook > CAS DataBase List > NUARIMOL
NUARIMOL
NUARIMOL
- CAS No.63284-71-9
- Chemical Name:NUARIMOL
- CBNumber:CB3375554
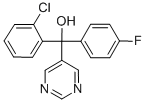
- Molecular Formula:C17H12ClFN2O
- Formula Weight:314.74
- MOL File:63284-71-9.mol
NUARIMOL Property
- Melting point 126°C
- Boiling point 475.2±40.0 °C(Predicted)
- Density 1.3255 (estimate)
- vapor pressure 1 x 10-5 Pa at 23 °C
- storage temp. 0-6°C
- solubility Chloroform (Slightly), Methanol (Slightly)
- Water Solubility 26 mg l-1 (pH 7) at 25 °C
- pka 11.37±0.29(Predicted)
- color White to Pale Yellow
- BRN 6223667
- FDA UNII ZU7K80U0CY
- EPA Substance Registry System Nuarimol (63284-71-9)
- Pesticides Freedom of Information Act (FOIA) Nuarimol
- UNSPSC Code 41116107
- NACRES NA.24
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H319
- Precautionary statements P264-P270-P280-P301+P312-P305+P351+P338-P337+P313
NUARIMOL Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 31116
- Product name : Nuarimol
- Purity: PESTANAL?, analytical standard
- Packaging: 100MG
- Price: ₹4156.8
- Updated: 2022/06/14
- Buy: Buy
NUARIMOL Chemical Properties,Usage,Production
- Uses Nuarimol is used in the preparation of difluoroethyl-containing heterocyclic compounds useful as antiviral agents and fungicides. Also used as a substance in the modelling inhibition of avian aromatase by azole pesticides.
- Uses Nuarimol is a systemic fungicide with both curative and protective activity. It controls a wide range of pathogenic fungi, e.g. Cercosporella spp., Septoria spp., Ustilago spp., powdery mildews, leaf spot, etc. in cereals (as a foliar spray and as a seed treatment), powdery mildews on pome fruits, stone fruits, vines, cucurbits and other crops and scab on apples.
- Metabolic pathway Photolytic degradation is the primary dissipation mechanism of nuarimol in the environment. Major degradation reactions observed on plants/soil surfaces and water include hydroxylation of the phenyl groups, oxidation of the carbinol carbon atom, dehalogenation and carbinol dehydroxylation (Scheme 1). The primary metabolic pathway of nuarimol in rats involves mainly aryl hydroxylation (Scheme 2).
-
Degradation
Nuarimol is stable to hydrolytic degradation when maintained in sterile
buffered solutions (pH 3,6 and 9) in the dark at 52 °C (Saunders, 1977). It
was readily degraded in distilled water via photolysis. The photolytic
DT50 of nuarimol was approximately 1 hour. Numerous photoproducts
were observed; however, no structural characterisation information was
reported (Zornes and Donoho, 1978).
Nuarimol was extensively photodegraded on solid surfaces. More than 80 photodegradation products were observed when nuarimol was exposed to sunlight on a stainless steel surface for up to 150 hours (Althaus, 1980a). All photoproducts were formed at very low levels (less than 3% each). The structures of 23 photoproducts were identified. An abbreviated photodegradation pathway of nuarimol (based on products accounting for 1% or greater) is presented in Scheme 1. These products were generated from the following reactions: aryl hydroxylation of the chlorophenyl and fluorophenyl moieties (to yield 2, 3), cleavage of the pyrimidine ring and the oxidation of the carbinol carbon atom (4,5) and dehydroxylation of the carbinol moiety (6). Carboxylic acid fragments from the phenyl(7,8,9) and pyrimidine moieties (10), resulting from the cleavage of the parent phenyl and pyrimidine linkages, were also observed.
NUARIMOL Preparation Products And Raw materials
Raw materials
Preparation Products
Global(64)Suppliers
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Henan Alfa Chemical Co., Ltd
- Tel: +8615838112936
- Email:alfa10@alfachem.cn
- Country:China
- ProdList:13194
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
SUZHOU SENFEIDA CHEMICAL CO.,LTD
- Tel:+86-0512-83500002<br/>+8618501500038
- Email:3899766280@qq.com
- Country:China
- ProdList:26362
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39927
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
63284-71-9, NUARIMOLRelated Search:
- Pyrimidines
- Pesticides&Metabolites
- N-PAlphabetic
- N
- Fungicides
- Alpha sort
- NJ - NZPesticides
- 杀菌剂类
- 杀虫剂
- 有机氯杀虫剂
- C17H12ClFN2O
- 甲苯中氟苯嘧啶醇
- 甲苯/丙酮中氟苯嘧啶醇
- 2-氯-4-氟-Α-(嘧啶-5-基)二苯基甲醇
- 纽瑞莫尔
- 氟苯嘧啶醇/噻菌醇
- NUARIMOL@1000 ΜG/ML IN ACETONE
- 氟苯嘧啶醇(噻菌醇) 标准品
- NUARIMOL SOLUTION
- 噻菌醇(氟苯嘧啶醇)
- 噻菌醇
- (±)2-氯-4′-氟-Α-(嘧啶-5-基)二苯基甲醇
- 氟苯嘧啶醇
- 5-(2-氯-4'-氟二苯甲基)-4-羟基嘧啶
- 63284-71-9
- Phenobarbital Impurity 20
- Nuarimol 100 μg/ml Ethyl acetate
- Nuarimol 100 μg/ml Acetonitrile
- Nuarimol 10.0 μg/ml Cyclohexane
- L15660000CY NuarimoL10μg/mLin Cyclohexane
- C15660000 Nuarimol
- 5-Pyrimidinemethanol, α-(2-chlorophenyl)-α-(4-fluorophenyl)-
- Trimidal @100 μg/mL in MeOH
- Nuarimol Solution
- Nuarimol@1000 μg/mL in Acetone
- 5-[(2-chlorophenyl)-(4-fluorophenyl)methyl]-1H-pyrimidin-6-one
- guantlet
- el2289
- el228
- alpha-(2-chlorophenyl)-alpha-(4-fluorophenyl)-5-pyrimidinemethanol
- alpha-(2-chlorophenyl)-alpha-(4-fluorophenyl)-5-pyrimidinemethano
- 5-(2-chloro-4’-fluorobenzhydryl)-4-hydroxypyrimidine
- 2-chloro-4’-fluoro-alpha-(pyrimidin-5-yl)diphenylmethanol
- 2-chloro-4’-fluoro-alpha-(pyrimidin-5-yl)benzhydrylalcohol
- 2-Chloro-4'-fluoro-alpha-(pyrimidin-5-yl)benz hydroxylalcohol
- NUARIMOL PESTANAL<TM>
- C11185
- TRIMIDAL STANDARD
- nuarimol (bsi,iso,ansi)
- Trimiol
- (±)α-(2-chlorophenyl)-α-(4-fluorophenyl)-5-pyrimidinemethanol
- (±)2-chloro-4′-fluoro-α-(pyrimidin-5-yl)benzhydrylalcohol
- triminol
- trimifruitsc
- murox
- (+-)-2-chloro-4’-fluoro-alpha-(pyrimidin-5-yl)benzhydrylalcohol
- TRIMIDAL
- NUARIMOL