ChemicalBook > CAS DataBase List > Ribociclib
Ribociclib
Ribociclib
- CAS No.1211441-98-3
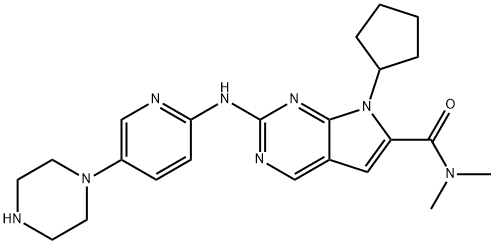
- Chemical Name:Ribociclib
- CBNumber:CB32677045
- Molecular Formula:C23H30N8O
- Formula Weight:434.54
- MOL File:1211441-98-3.mol
Ribociclib Property
- Boiling point 730.8±70.0 °C(Predicted)
- Density 1.39±0.1 g/cm3(Predicted)
- vapor pressure 0-0Pa at 20-25℃
- storage temp. -20°C
- solubility Soluble in DMSO (up to 10 mg/ml)
- form solid
- pka 8.67±0.10(Predicted)
- color Off-white
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months.
- LogP -0.8-2.3 at 20℃ and pH4-11
- FDA UNII TK8ERE8P56
- NCI Drug Dictionary Kisqali
- ATC code L01EF02
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P280-P305+P351+P338
Ribociclib Chemical Properties,Usage,Production
- Appearance yellow Solid
-
Description
LEE011 is a cyclin-
dependent kinase (CDK) inhibitor that targets cyclin D1/CDK4 and cyclin D3/CDK6 at nanomolar concentrations. It inhibits retinoblastoma protein phosphorylation, which prevents CDK-mediated G1-S phase transition, arresting the cell cycle in the G1 phase, suppressing DNA synthesis, and inhibiting cancer cell growth. LEE011 has been shown to reduce proliferation in 12 of 17 human neuroblastoma- derived cell lines by inducing cytostasis (mean IC50 = 306 nM in sensitive lines). -
Characteristics
Class: serine/threonine protein kinase
Treatment: Breast cancer
Oral bioavailability = 66%
Elimination half-life = 32 h
Protein binding = 70% - Uses LEE 011 can be used in biological study of kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer.
- Mechanism of action Ribociclib is a dual cdk4/cdk6 inhibitor. Induces G1 phase cell cycle arrest and senescence in neuroblastoma cell lines (IC50 = 306 nM). Delays tumor growth in vivo.
-
Clinical Use
Protein kinase inhibitor:
Treatment of breast cancer -
Clinical Use
Ribociclib was first approved in 2017 as the second CDK4/6 (cyclin-dependent kinase) inhibitor in combination with aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2?) advanced or metastatic breast cancer. In 2018, its label was expanded to pre/perimenopausal women with HR+/HER2? advanced or metastatic breast cancer.
- target CDK4
-
Drug interactions
Potentially hazardous interactions with other drugs
The concomitant use of strong CYP3A4 inhibitors including, but not limited to, the following must be avoided: clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil and voriconazole.
The concomitant use of strong CYP3A4 inducers may therefore lead to decreased exposure and consequently a risk for lack of efficacy. The concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John's wort.
Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pravastatin, rosuvastatin and metformin.
Co-administration of Kisqali with medicinal products with a known potential to prolong the QT interval such as anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol), and other medicinal products that are known to prolong the QT interval (including, but not limited to, chloroquine, halofantrine, clarithromycin, haloperidol, methadone, moxifloxacin, pimozide and intravenous ondansetron) should be avoided. -
Metabolism
Ribociclib is hepatically metabolised via CYP3A4 by
oxidation. Ribociclib was the main circulating drug in
plasma (44%). The major circulating metabolites included
metabolite M13 (CCI284, N-hydroxylation), M4 (LEQ803,
N-demethylation), and M1 (secondary glucuronide).
Clinical activity of ribociclib was due mainly to parent drug,
with negligible contribution from circulating metabolites.
Unchanged drug accounted for 17.3% and 12.1% of
the dose in faeces and urine, respectively. Metabolite
LEQ803 represented approximately 13.9% and 3.74%
of the administered dose in faeces and urine, respectively.
Numerous other metabolites were detected in both faeces
and urine in minor amounts (≤2.78%).
Ribociclib and its metabolites are eliminated mainly via faeces (69.1%), with a small contribution from the renal route (22.6%). - storage Store at -20°C
- References 1) Rader?et al.?(2013),?Dual CDK4/6 inhibition induces cell-cycle arrest and senescence in neuroblastoma; Clin. Cancer Res.?19?6173 2) Kim?et al. (2013),?LEE011: an orally bioavailable, selective small-molecule inhibitor of CDK4/6 – reactivating Rb in cancer; Mol. Cancer Ther.?12?PR02 3) Tripathy?et al.?(2017),?Ribociclib(LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors; Clin. Cancer Res. March 28, 201
Ribociclib Preparation Products And Raw materials
Raw materials
Preparation Products
Global(241)Suppliers
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
TianYuan Pharmaceutical CO.,LTD
- Tel:+86-755-23284190 13684996853
- Email:sales@tianpharm.com
- Country:CHINA
- ProdList:304
- Advantage:58
-
Supplier:
Biochempartner
- Tel:0086-13720134139
- Email:candy@biochempartner.com
- Country:CHINA
- ProdList:965
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Shochem(Shanghai) Co.,Ltd
- Tel:86-21-50800795
- Email:info@shochem.com
- Country:CHINA
- ProdList:288
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
Shanghai Jinghao Pharmaceutical Co.,Ltd
- Tel:+86-21-68900963
- Email:sales@jinghaopharma.com
- Country:CHINA
- ProdList:153
- Advantage:58
Related articles
1211441-98-3, RibociclibRelated Search:
- Inhibitors
- 化学试剂
- 抑制剂
- 化工原料
- 医药化工
- FDA批准的配体
- 医用原料
- 医药原料
- 普通产品
- 杂质对照品
- 医药原料药
- 小分子抑制剂
- 小分子抑制剂,天然产物
- C23H30N8O
- 瑞柏司可相关杂质
- LEE011/1211441-98-3
- 瑞博西尼,10 MM DMSO 溶液
- 瑞博西利(碱基)
- 瑞博西尼碱基
- 利波西利
- 琥珀酸利柏西利
- 核糖体
- 瑞博西尼杂质
- 瑞博西尼BASE
- 瑞博西利
- 7-环戊基-N,N-二甲基-2-((5-(哌嗪-1-基)吡啶-2-基)氨基)-7H-吡咯并[2,3-D]嘧啶-6-甲酰胺
- 7-环戊基-N,N-二甲基-2-[[5-(1-哌嗪基)-2-吡啶基]氨基]-7H-吡咯并[2,3-D]嘧啶-6-甲酰胺
- LEE011 瑞柏司可里布
- 瑞柏司可里布
- LEE011 瑞柏司可里布 50MG
- 1211441-98-3
- Ribociclib, 10 mM in DMSO
- Ribociclib base
- Cyclin dependent kinase,LEE 011,Ribociclib,inhibit,Inhibitor,LEE-011,CDK
- 7-Cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide
- LEE011; LEE-011. LEE 011; RIBOCICLIB; KISQALI.
- LEE-011. LEE 011
- Kisqali.
- LEE-011,Ribociclib,LEE011
- Ribociclib-d8
- LEE 011; LEE-011;RIBOCICLIB;LEE011
- CS-1001
- LEE01
- 7H-Pyrrolo[2,3-d]pyrimidine-6-carboxamide, 7-cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyri
- 7-cyclopentyl-N,N-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo[2,3-d]pyrimidine-6-carboxamide
- LEE11
- Ribociclib Lee011 Powder
- Ribociclib USP/EP/BP
- Ribociclib
- 7-Cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidi
- Ribociclib 7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide
- 7H-Pyrrolo[2,3-d]pyrimidine-6-carboxamide, 7-cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-
- LEE-011, Ribociclib
- 7-CYCLOPENTYL-2-(5-PIPERAZIN-1-YL-PYRIDIN-2-YLAMINO)-7H-PYRROLO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID DIMETHYLAMIDE
- LEE011, >=98%
- LEE 011 7-Cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide
- 7-Cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide LEE 011
- 7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide