ChemicalBook > CAS DataBase List > Remifentanil Hydrochloride
Remifentanil Hydrochloride
Remifentanil Hydrochloride
- CAS No.132539-07-2
- Chemical Name:Remifentanil Hydrochloride
- CBNumber:CB31105671
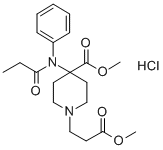
- Molecular Formula:C20H28N2O5.ClH
- Formula Weight:412.911
- MOL File:132539-07-2.mol
Remifentanil Hydrochloride Property
- Melting point 195-197°C
- Flash point 9°C
- storage temp. 2-8°C
- solubility PBS (pH 7.2): 10 mg/ml
- form A neat solid
- CAS DataBase Reference 132539-07-2
- FDA UNII 5V444H5WIC
- NCI Drug Dictionary remifentanil hydrochloride
Safety
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H225-H301+H311+H331-H370
- Precautionary statements P210-P260-P280-P301+P310-P311
Remifentanil Hydrochloride Chemical Properties,Usage,Production
- Description Ultiva was launched in Germany and the US for use in general anesthesia and immediate post-operative pain management. This compound can be prepared via the Michael addition of a 4,4-disubstituted piperidine to methyl acrylate. It behaves as a specific μ-opiod agonist that is about 30 times more potent than its chemical relative alfentanil. While its pharmacodynamic properties are similar to other μ agonists, remifentanil has unique pharmacokinetic properties. It has a rapid onset (1.6 min) and a rapid offset (5.4 min) independent of duration of administration. This has been attributed to its rapid catabolism by general esterases to the corresponding acrylic acid derivative. The metabolite has 0.01 to 0.0005 times the activity of remifentanil and is excreted via the kidneys (80% recovery).
- Chemical Properties White Solid
- Originator Glaxo Wellcome (UK)
- Uses Labelled synthetic mu-opioid agonist. This is a controlled substance (opiate)
- brand name Ultiva (Abbott).
- Biochem/physiol Actions Remifentanil hydrochloride is a mu opioid receptor agonist, anesthetic, and analgesic compound. Remifentanil was developed as an ultra-short-acting mu opioid receptor agonist with improved pharmacodynamic properties.
- Pharmacokinetics Remifentanil is much like alfentanil in its pharmacodynamic effects. It is a selective μ opioid agonist with 15 to 20 times greater potency than alfentanil. Remifentanil has an onset of action of 1 to 3 minutes when given intravenously. Its unique property is its rapid offset of action, which is independent of the duration of administration of the compound. Thus, it is very useful for titration of antinociceptive effect, followed by a rapid and predictable recovery time of 3 to 5 minutes. The short duration of action is a result of the ester group, which has been rationally designed into the substituent on the piperidine nitrogen. This ester group is rapidly hydrolyzed to the inactive carboxylic acid by serum and tissue esterases, making the drug's duration of action essentially independent of the liver or renal function of the patient. Remifentanil is used extensively for analgesia associated with general anesthesia procedures. It often is used in combination with injectable general anesthetic agents, such as midazolam or propofol.
Remifentanil Hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
132539-07-2, Remifentanil HydrochlorideRelated Search:
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Aromatics
- C20H28N2O5ClH
- C20H29ClN2O5
- 132539-07-2
- 33,72-difluoro-N,N′-dimethyl-5-oxo-2,8-dioxa-4,6-diaza-1(4),9(4)-dipyridina-3(1,4),7(1,4)-dibenzenanonaphane-12,92-dicarboxamide
- Remifentanil HydrochlorideQ: What is Remifentanil Hydrochloride Q: What is the CAS Number of Remifentanil Hydrochloride Q: What is the storage condition of Remifentanil Hydrochloride Q: What are the applications of Remifentanil Hydrochloride
- Remifentanil (hydrochloride) (CRM)
- remifnetanil hydrochloride
- REMIFENTANIL HYDROCHLORIDE SOLUTION
- WFBMIPUMYUHANP-UHFFFAOYSA-N
- Unii-5V444H5wic
- Remifentanil HCl
- 1-Piperidinepropanoic acid, 4-(methoxycarbonyl)-4-((1-oxopropyl)phenylamino)-, methyl ester, monohydrochloride
- GI 87084B
- Ultiva
- Remifentanyl Hydrochloride
- GI 87084
- 4-(Methoxycarbonyl)-4-[(1-oxopropyl)phenylamino]-1-piperidinepropanoic Acid Methyl Ester Hydrochloride
- Remifentanil Hydrochloride