ChemicalBook > CAS DataBase List > SPINOSAD
SPINOSAD
SPINOSAD
- CAS No.131929-60-7
- Chemical Name:SPINOSAD
- CBNumber:CB2416797
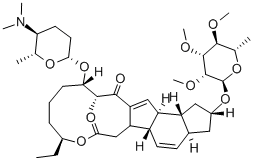
- Molecular Formula:C41H65NO10
- Formula Weight:731.96
- MOL File:131929-60-7.mol
SPINOSAD Property
- Boiling point 801.5±65.0 °C(Predicted)
- Density 1.16±0.1 g/cm3(Predicted)
- vapor pressure Spinosyn A: 3.2 x 10-8 Pa
Spinosyn D: 2.1 x 10-8 Pa - storage temp. Store at -20°C
- solubility Soluble in DMSO
- form solid
- pka Spinosyn A: 8.1 (base); Spinosyn D: 7.8 (base)
- Water Solubility Spinosyn A: 290 mg l-1 (pH 5); Spinosyn D: 29 mg l-1 (pH 5)
- color White to off-white
- InChIKey SRJQTHAZUNRMPR-XOBNMGJWNA-N
- EWG's Food Scores 1
- FDA UNII OY0L59V61N
- Pesticides Freedom of Information Act (FOIA) Spinosad
- EPA Substance Registry System Spinosyn A (131929-60-7)
Safety
- RIDADR :3077
- HazardClass :9
- PackingGroup :III
- Hazardous Substances Data :131929-60-7(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H410
- Precautionary statements P273-P391-P501
SPINOSAD Chemical Properties,Usage,Production
- Uses Spinosyn A is the major component of a complex of unusual, hydrophobic macrocyclic lactones isolated from Saccharopolyspora spinosa in 1991. The 12-membered macrocyclic lactone is fused to form a rare 12-5-6-5 tetracyclic ring system, with the macrocycle and the terminal cyclopentane bearing glycosides. Spinosyn A is a potent insecticide for crop pathogens and ectoparasite control on animals. The spinosyns have a unique mechanism of action involving disruption of nicotinic acetylcholine receptors.
- Definition ChEBI: A spinosyn in which the sugar amino and hydroxy groups are globally methylated. One of the two active ingredients of spinosad.
- Biological Activity spinosyn a is an insect nicotinic acetylcholinesterase receptors (nachrs) agonist and potent insecticide.the spinosyns are a family of macrolide natural products produced by the soil microorganism saccharopolyspora spinosa. spinosyn a is identified as a naturally-occurring macrocyclic lactone that is a potent insecticide.
-
Veterinary Drugs and Treatments
For the prevention and treatment of fleas for one month on dogs 14 weeks of age and older.
Spinosad is a group 5 nicotinic acetylcholine receptor agonist, that causes involuntary muscle contractions and tremors secondary to motor neuron activation. Prolonged exposure causes paralysis and flea death. Flea death begins within 30 minutes of dosing and in 4 hours is complete. Spinosad does not interact with bindings sites of other insecticidal agents (GABA-ergic or nicotinic). - in vitro the mixture of spinosyns a and d, a commercial insecticide tracertm (dowagrosciences), is useful against various crop pests such as tobacco budworm. it was found that the deoxy analogs of spinosyns a were more potent insecticides than their respective parent factor. moreover, the 2’-desmethoxy analogs of spinosyns a showed insecticidal potency against h. virescens greater than that of spinosyns a and d, suggesting that polarity was not well tolerated. furthermore, the activity of 3'-deoxy spinosyn j was about the same as spinosyn a, and the activity of 2'-deoxy spinosyn h was found to be slightly greater than that of spinosyn a [1].
- Metabolic pathway Spinosad consists of two major components, namely psinosyns A (ca 85%) and D (ca 15%). When either 14C-spinosyn A or -spinosyn D is applied in the soil under aerobic conditions, the major degradation product of spinosyn A is spinosyn B, resulting from demethylation on the forosamine sugar. Other degradation products are hydroxylation products of psinosyns A and B, probably on the aglycone portion of the molecule.
- Degradation Spinosad is relatively stable in water with no observed decomposition between pH 5 and 7. Measured DT50 values at pH 9 were 200 and 259 days, respectively, for spinosyns A and D (Saunders and Bret, 1997). Aqueous photolysis was very rapid with a DT50 of < 1 day in pH 7 buffered water at 25 °C (DowElanco, 1996). Spinosad was rapidly dissipated and degraded in an outdoor mesocosm study and on plant surfaces (Saunders and Bret, 1997).
- Toxicity evaluation Acute oral LD50 for rats: 2,000-5,000 mg/kg
- References [1] l. c. creemer, h. a. kirst, j. w. paschal, et al. synthesis and insecticidal activity of spinosyn analogs functionally altered at the 2'-,3'- and 4'-positions of the rhamnose moiety. j.antibiot.(tokyo) 53(2), 171-178 (2000).
SPINOSAD Preparation Products And Raw materials
Raw materials
Preparation Products
Global(225)Suppliers
-
Supplier:
Qingdao Trust Agri Chemical Co.,Ltd
- Tel: +8613573296305
- Email:aroma@qdtrustagri.com
- Country:China
- ProdList:301
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Wuhan Boyuan Import & Export Co., LTD
- Tel: +8615175982296
- Email:Mike@whby-chem.com
- Country:China
- ProdList:33
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3882
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Shaanxi Haibo Biotechnology Co., Ltd
- Tel: +undefined18602966907
- Email:qinhe02@xaltbio.com
- Country:China
- ProdList:997
- Advantage:58
-
Supplier:
Henan Suikang Pharmaceutical Co.,Ltd.
- Tel:+86-18239973690<br/>+86-18239973690
- Email:sales@suikangpharm.com
- Country:China
- ProdList:311
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel: +8613288715578
- Email:angelia@hbmojin.com
- Country:China
- ProdList:750
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883<br/>+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
131929-60-7, SPINOSADRelated Search:
- Emamectin Bensultap BEAUVERICIN Thiocyclam Abamectin Emamectin SPINOSAD 2-Isopropyl-5-methyl-2-hexenal METHYL-CIS-4-DECENOATE SPINOSAD 4,4-Diethoxy-N,N-dimethyl-1-butanamine 4-(N,N-Dimethylamino)butanal Dimethyl Acetal ethyl 5-oxodecanoate SPINOSAD 100MG [R] SPINOSAD SOLUTION 100UG/ML IN TOLUENE 1ML SPINOSAD SOLUTION 100UG/ML IN ACETONITRILE 1ML ETHYL 6-METHYL-5-OXOHEPTANOATE octyl isononanoate
- 原药
- 农用原药
- 产品
- 农用原药-原药
- 医用原料
- 抑制剂
- 农用原粉
- 农药原料,农药中间体
- 医药
- 精细化工原料
- 化学试剂
- 化学原料
- 化学试剂-农药原料
- 其他类
- 原料药
- 化工原料
- 兽用原料
- 农药原料
- 农药原料
- 原料药
- 添加剂
- 添加剂
- 有机化工原料
- 农药原料药
- 微生物代谢物
- 杀虫剂
- 有机氯杀虫剂
- 医药原料药
- 微生物杀虫剂
- 杀虫(螨)剂
- 农药
- 多杀菌素AC42H67NO16
- C41H65NO10
- 68316-95-8
- 929-60-7
- 多杀霉素 A,10 MM DMSO 溶液
- 多杀菌素90%
- 乙腈中多杀霉素A
- 甲醇中多杀霉素溶液标准物质
- 多杀霉素(多杀霉素A)检测标准品
- 多杀霉素溶液
- 多棘寡糖/多杀菌素
- 多杀菌素A/D
- (2R,3AS,5AR,5BS,9S,13S,14R,16AS,16BR)-2-[(6-脱氧-2,3,4-三-O-甲基-Α-L-吡喃甘露糖基)氧基]-13-[[(2R,5S,6R)-5-(二甲基氨基)四氢-6-甲基-2H-吡喃-2-基]氧基]-9-乙基代-2,3,3A,5A,5B,6,9,10,11,12,13,14,16A,16B-十四氢-14-甲基-1H-苯并二茚并[3,2-D]氧杂环十二碳-7,15-二酮
- 甲醇中多杀霉素A溶液,1000ΜG/ML
- SPINOSYN A SOLUTION
- 多杀霉素A溶液,1000PPM
- 多杀菌素A
- 多杀菌素
- 多杀霉素 A
- 多杀霉素
- 多杀霉素A溶液, 100PPM
- 131929-60-7
- Spinosyn A, 10 mM in DMSO
- Dasatinib Impurity 86
- Spinosad A (Spinosyn A) in Acetonitrile
- Spinosad A (Spinosyn A)
- (-)-Spinosyn A