ChemicalBook > CAS DataBase List > Vinflunine
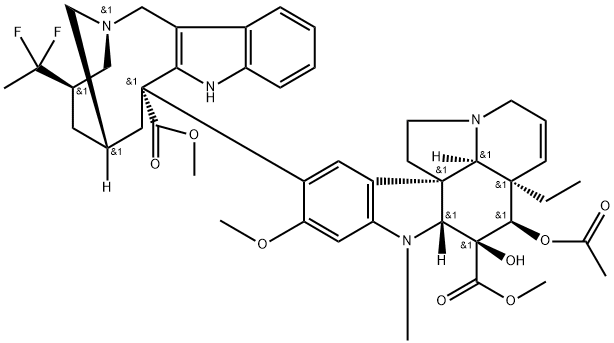
Vinflunine
Vinflunine
- CAS No.162652-95-1
- Chemical Name:Vinflunine
- CBNumber:CB2358406
- Molecular Formula:C45H54F2N4O8
- Formula Weight:816.94
- MOL File:162652-95-1.mol
Vinflunine Property
- Density 1.39±0.1 g/cm3(Predicted)
- pka 11.36±0.60(Predicted)
- CAS DataBase Reference 162652-95-1(CAS DataBase Reference)
- FDA UNII 5BF646324K
- NCI Dictionary of Cancer Terms vinflunine
- NCI Drug Dictionary vinflunine
- ATC code L01CA05
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Vinflunine Chemical Properties,Usage,Production
- Description Vinflunine (also referred to as PM391) is a semisynthetic analog of the natural vinca alkaloids vinblastine and vincristine that was approved by the European Medicines Agency (EMEA) in 2009 for the treatment of adult patients with advanced or metastatic transitional cell carcinoma of the urothelial tract after failure of a prior platinum-containing regimen. Vinflunine is synthesized from anhydrovinblastine in a superacid media (HF-SbF5) and in the presence of a chlorinated solvent like CHCl3 or CCl4.
- Originator Pierre Fabre (France)
- Definition ChEBI: An organic heteropentacyclic compound and an organic heterotetracyclic compound that is vinorelbine in which the tetrahydropyridine moiety of the heterotetracyclic part of the molecule has been redced to the corresponding piperidine, and in which the ethy group attached to this ring has been replaced by a 1,1-difluoroethyl group.
- brand name Javlor
-
Clinical Use
Antineoplastic agent (vinca alkaloid):
Treatment of advanced or metastatic bladder cancer -
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: possible increased risk of ventricular arrhythmias with delamanid; concentration possibly reduced by rifampicin - avoid.
Antidepressants: concentration possibly reduced by St Johns wort - avoid.
Antifungals: concentration increased by ketoconazole and possibly itraconazole (increased risk of neurotoxicity) - avoid.
Antimalarials: avoid with piperaquine with artenimol.
Antipsychotics: avoid with clozapine (increased risk of agranulocytosis).
Antivirals: concentration possibly increased by ritonavir - avoid.
Grapefruit juice: concentration of vinflunine increased - avoid. - Metabolism Metabolised by the cytochrome CYP3A4 isoenzyme, except for 4-O-deacetylvinflunine (DVFL), the only active metabolite and main metabolite in blood which is formed by multiple esterases. Excretion occurs via the faeces (about two-thirds) and the urine (about one-third).
Vinflunine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(101)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
NanJing Spring & Autumn Biological Engineering CO., LTD.
- Tel: +8613815430202
- Email:sale02@cqherb.com
- Country:CHINA
- ProdList:376
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Dideu Industries Group Limited
- Tel:+86-29-89586680<br/>+86-15129568250
- Email:1026@dideu.com
- Country:China
- ProdList:22854
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15349
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
162652-95-1, VinflunineRelated Search:
- (R)-3-AMINO-4-(3-METHYLPHENYL)BUTANOIC ACID HYDROCHLORIDE 2,4-Dipentylphenol 3-AMINO-4-(4-METHOXY-PHENYL)-BUTYRIC ACID 2-TERT-BUTYL-1H-INDOLE 4-PHENYLCYCLOHEXANOL α-(p-Aminophenyl)butyric acid (2S)-2,3-Dihydroxy-2-methyl-propanoic Acid Methyl Ester 90% TRANS-4-AMINOCYCLOHEXANECARBOXYLIC ACID 4-AMINOCYCLOHEXANECARBOXYLIC ACID (R)-(-)-2-HYDROXY-4-PHENYLBUTYRIC ACID Vinflunine INDOLE-2-ACETIC ACID 3,3,5,5-TETRAMETHYLCYCLOHEXANOL 1-HYDROXY-CYCLOHEXANECARBOXYLIC ACID L-LEUCIC ACID (2-METHOXY-5-METHYLPHENYL)ACETIC ACID 3-PHENETHYL-PIPERIDINE (2S,3R)-3-AMINO-2-HYDROXY-4-PHENYLBUTYRIC ACID HYDROCHLORIDE
- Miscellaneous Natural Products
- 标准品 -中药标准品
- 原料药
- 医药原料药 科研原料
- 植物提取物
- 标准品
- 医药中间体
- 科研试剂
- C45H54F2N4O8
- 长春氟宁(治疗膀胱癌)
- 长春氟宁
- 酒石酸长春氟宁
- 162652-95-1
- vinflunine(97%)
- Aspidospermidine-3-carboxylicacid,4-(acetyloxy)-6,7-didehydro-15-[(2R,4R,6S,8S)-4-(1,1-difluoroethyl)-1,3,4,5,6,7,8,9-octahydro-8-(methoxycarbonyl)-2,6-methano-2H-azecino[4,3-b]indol-8-yl]-3-hydroxy-1
- Vinflunine (F12158)
- Vinflunine USP/EP/BP
- Aspidospermidine-3-carboxylic acid, 4-(acetyloxy)-6,7-didehydro-15-[(2R,4R,6S,8S)-4-(1,1-difluoroethyl)-1,3,4,5,6,7,8,9-octahydro-8-(methoxycarbonyl)-2,6-methano-2H-azecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methyl-, methyl ester, (2β,3β,4β,5α,12R,19α)-
- Vinflunine 162652-95-1
- Vinflunine Tartrate
- 4'-Deoxy-20',20'-difluoro-8'-norvincaleukoblastine
- 4'-Deoxy-20',20'-difluoro-5'-norvincaleukoblastine
- 20',20'-Difluoro-3',4'-dihydrovinorelbine
- VINFLUNINE